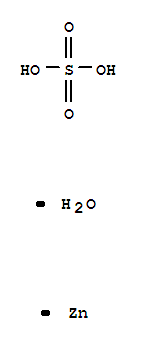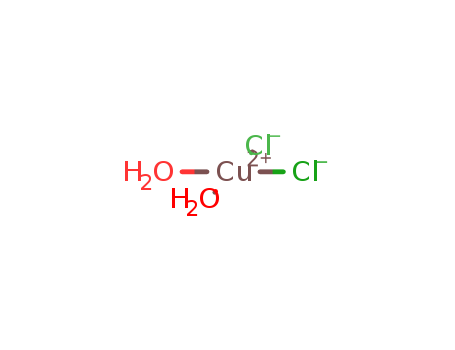
10125-13-0
- Product Name:Copper(II) chloride dihydrate
- Molecular Formula:CuCl2.2(H2O)
- Purity:99%
- Molecular Weight:170.483
Product Details;
CasNo: 10125-13-0
Molecular Formula: CuCl2.2(H2O)
Appearance: moist blue crystals.
Buy High Grade Copper(II) chloride dihydrate 10125-13-0 Efficient Delivery,Sale 10125-13-0
- Molecular Formula:Cl2CuH4O2
- Molecular Weight:170.483
- Appearance/Colour:moist blue crystals.
- Melting Point:100 °C (dec.)(lit.)
- Boiling Point:100 °C at 760 mmHg
- PSA:18.46000
- Density:2.54 g/cm3
- LogP:1.24790
Copper(II) chloride dihydrate(Cas 10125-13-0) Usage
|
Description |
Copper (II) chloride dihydrate is used in the preparation of copper oxychloride and as a catalyst in many organic chlorination reactions such as vinyl chloride and 1,2-dichloroethane. Copper chloride dihydrate may be also used as catalyst to cleave tetrahydropyranyl (THP) ethers and t-butyldimethylsilyl (TBDMS) ethers and to chemoselectively hydrolyze semicarbazones to carbonyl compounds. Solution of copper (II) chloride is used for plating on aluminum and for tinting germanium and tin. |
|
Uses |
Copper(II) chloride is used as a mordant in dyeing and printing of fabrics; as an ingredient of isomerization and cracking catalysts; and as a desulfurizing and deodorizing agent in petroleum industry. Other important applications are in copper plating of aluminum; preparation of copper standard solutions; test for molybdenum; in tinting-baths for iron and tin; in pigments for ceramics and glasses; as a fixer and desensitizer reagent in photography; in mercury extraction from ores; in laundry-marking and invisible inks; and in manufacture of several copper salts. |
|
Preparation |
Copper(II) chloride may be synthesized by heating elemental copper with chlorine: Cu + Cl2 CuCl2 Alternatively, it may be prepared by treating copper carbonate with hydrochloric acid followed by crystallization: CuCO3 + 2HCl → CuCl2 + CO2 + H2O In the above preparation, the hydrate of the salt crystallizes, precipitates, and may be dehydrated by heating under vacuum. |
|
Chemical Properties |
Moist blue crystals. |
InChI:InChI=1/2ClH.Cu.H2O/h2*1H;;1H2/q;;+2;/p-2/rCl2Cu.H2O/c1-3-2;/h;1H2
Relevant Products
-
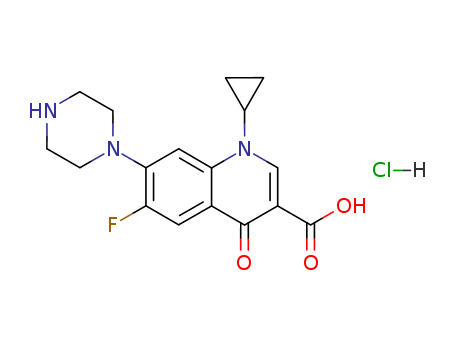
Ciprofloxacin HCl
CAS:93107-08-5
-
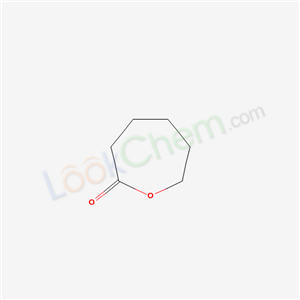
Poly(ε-caprolactone)
CAS:24980-41-4
-
Zinc sulfate monohydrate
CAS:7446-19-7