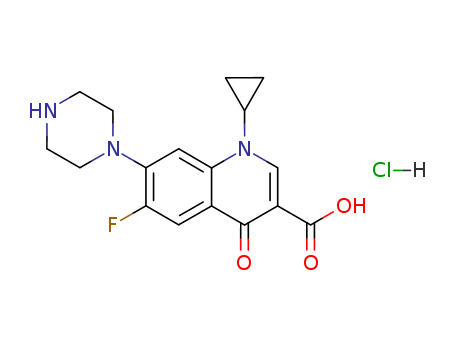
93107-08-5
- Product Name:Ciprofloxacin HCl
- Molecular Formula:C17H18FN3O3.HCl
- Purity:99%
- Molecular Weight:367.808
Product Details;
CasNo: 93107-08-5
Molecular Formula: C17H18FN3O3.HCl
Appearance: white or light yellow crystalline powder
Buy Top Purity High Grade Ciprofloxacin HCl 93107-08-5 Cheap Price
- Molecular Formula:C17H18FN3O3.HCl
- Molecular Weight:367.808
- Appearance/Colour:white or light yellow crystalline powder
- Melting Point:>300 °C
- Boiling Point:581.8 °C at 760 mmHg
- Flash Point:305.6 °C
- PSA:74.57000
- LogP:2.77910
Ciprofloxacin HCl(Cas 93107-08-5) Usage
|
Description |
Ciprofloxacin hydrochloride is a synthetic second-generation quinolone antibacterial drug with potent bactericidal effects against various bacteria, including Enterobacter, Pseudomonas aeruginosa, Haemophilus influenzae, Neisseria gonorrhoeae, Streptococcus, Legionella, and Staphylococcus aureus. It inhibits bacterial DNA gyrase subunit A, preventing the cleavage and linking functions of the enzyme, thus hindering bacterial DNA replication. Ciprofloxacin differs from other quinolones due to a fluorine atom at the 6-position, a piperazine moiety at the 7-position, and a cyclopropyl ring at the 1-position. |
|
Uses |
Marketed as a broad-spectrum antibacterial agent, ciprofloxacin hydrochloride is highly effective against bacterial causes of enteritis. Additionally, it was the drug of choice for combating anthrax. Ciprofloxacin functions by inhibiting DNA gyrase and topoisomerase IV, crucial enzymes for bacterial replication. In radiation combined injury cases, it has shown immunomodulatory activities by reducing pro-inflammatory cytokines, inhibiting apoptosis and autophagy, and enhancing IL-3 production. |
|
Brand name |
Ciloxan (Alcon); Cipro (Bayer); Proquin (Esprit). |
|
Biological Activity |
FDA-approved for managing postexposure inhalational anthrax, ciprofloxacin demonstrates significant survival improvement in animals exposed to aerosolized Bacillus anthracis. In the USAMRIID rhesus monkey model, it achieves a maximum concentration (Cmax) of 1.74 μg/ml and a minimum concentration (Cmin) of 0.17 μg/ml, inhibiting the growth of B. anthracis with a MIC90 of 0.06 μg/ml. |
|
references |
[1]. fukumoto r, cary lh, gorbunov nv, et al. ciprofloxacin modulates cytokine/chemokine profile in serum, improves bone marrow repopulation, and limits apoptosis and autophagy in ileum after whole body ionizing irradiation combined with skin-wound trauma. plos one. 2013;8(3):e58389.[2]. drlica k, zhao x. dna gyrase, topoisomerase iv, and the 4-quinolones. microbiol mol biol rev. 1997 sep;61(3):377-92.[3]. meyerhoff a, albrecht r, meyer jm, et al. us food and drug administration approval of ciprofloxacin hydrochloride for management of postexposure inhalational anthrax. clin infect dis. 2004 aug 1;39(3):303-8. |
InChI:InChI=1/C17H18FN3O3.ClH/c18-13-7-11-14(8-15(13)20-5-3-19-4-6-20)21(10-1-2-10)9-12(16(11)22)17(23)24;/h7-10,19H,1-6H2,(H,23,24);1H
93107-08-5 Relevant articles
Preparation method of quinolone carboxylic acid derivative or phthalazinone carboxylic acid derivative
-
, (2021/10/27)
The invention belongs to the field of ph...
Advanced Continuous Flow Platform for On-Demand Pharmaceutical Manufacturing
Zhang, Ping,Weeranoppanant, Nopphon,Thomas, Dale A.,Tahara, Kohei,Stelzer, Torsten,Russell, Mary Grace,O'Mahony, Marcus,Myerson, Allan S.,Lin, Hongkun,Kelly, Liam P.,Jensen, Klavs F.,Jamison, Timothy F.,Dai, Chunhui,Cui, Yuqing,Briggs, Naomi,Beingessner, Rachel L.,Adamo, Andrea
, p. 2776 - 2784 (2018/02/06)
As a demonstration of an alternative to ...
Isolation and Characterization of Ciprofloxacin-HCL Crystals
A. P. Kakkar,Manmohan Singh &Arun Mendiratta
, Drug Development and Industrial Pharmacy Volume 23, 1997 - Issue 11
Three crystalline and one amorphous form of Ciprofloxacin-HCL were prepared and characterized by various instrumental techniques. The hygroscopicity, stability, and solubility of all the forms were also determined. Results indicated that all three crystalline and one amorphous form had distinctly different properties.
93107-08-5 Process route
-
-
C21H23BFN3O7

-
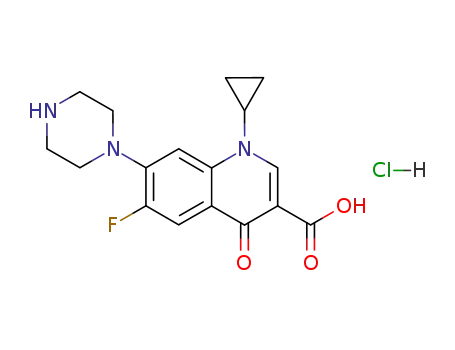
- 93107-08-5,86483-48-9
ciprofloxacin hydrochloride
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; In water; at 0 ℃; for 4h; pH=< 1;
|
92.4% |
-
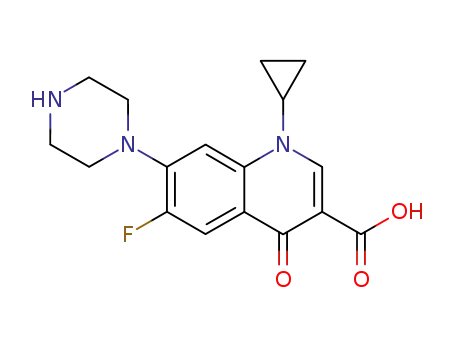
- 85721-33-1,178489-05-9,189257-90-7
ciprofloxacin

-

- 93107-08-5,86483-48-9
ciprofloxacin hydrochloride
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; In water; Heating;
|
98% |
|
With hydrogenchloride; In 1,4-dioxane; dichloromethane; at 20 ℃; for 1h;
|
95% |
|
With hydrogenchloride; In methanol; water; at 45 - 50 ℃; for 2h; Product distribution / selectivity;
|
93107-08-5 Upstream products
-
110-85-0
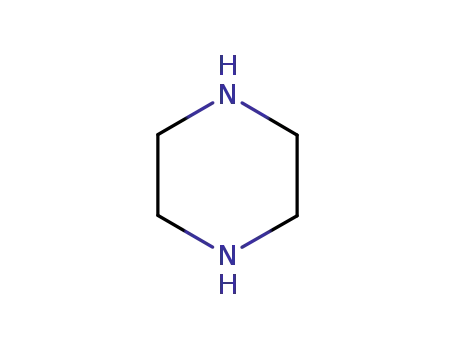
piperazine
-
93107-30-3
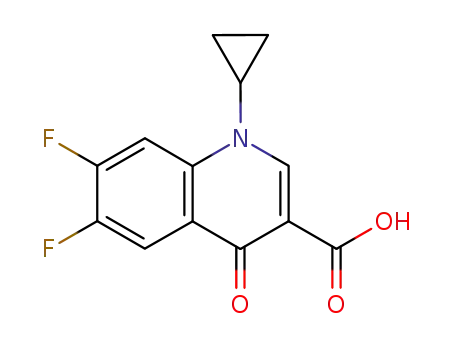
1-cyclopropyl-6,7-difluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid
-
85721-33-1

ciprofloxacin
-
86393-33-1
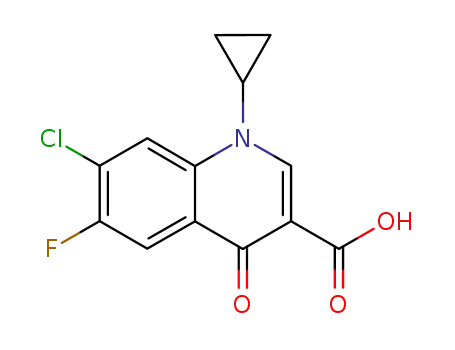
7-chloro-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-3-quinoline carboxylic acid
93107-08-5 Downstream products
-
93594-39-9
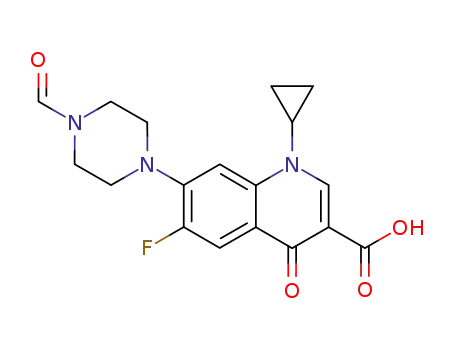
1-cyclopropyl-6-fluoro-7-(4-formylpiperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid
-
105093-21-8
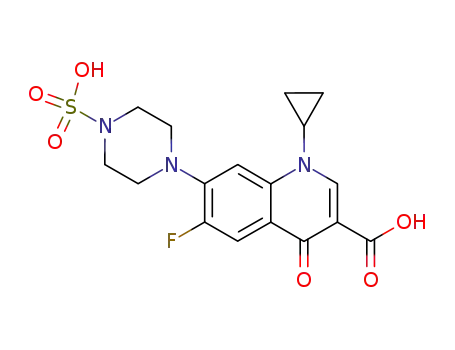
Sulfo-Ciprofloxacin
-
103222-12-4
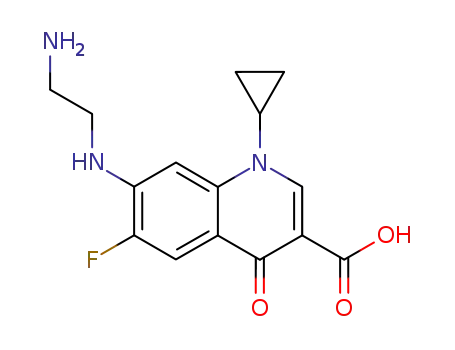
Desethyleneciprofloxacin
-
86393-33-1

7-chloro-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-3-quinoline carboxylic acid
Relevant Products
-
4F-MPH
CAS:1354631-33-6
-
Monosodium glutamate
CAS:32221-81-1
-
Semaglutide
CAS:910463-68-2