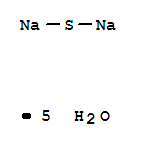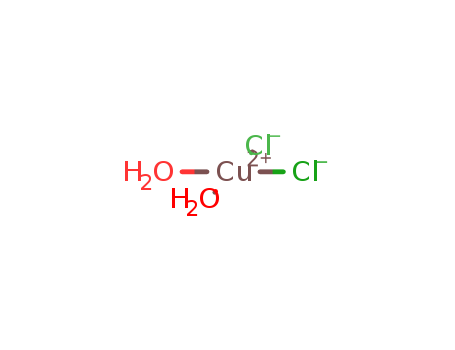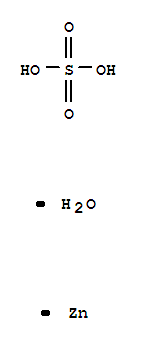
7446-19-7
- Product Name:Zinc sulfate monohydrate
- Molecular Formula:H2O5SZn
- Purity:99%
- Molecular Weight:179.49
Product Details;
CasNo: 7446-19-7
Molecular Formula: H2O5SZn
Appearance: white powder or granules
Buy Quality Zinc sulfate monohydrate 7446-19-7,Offer 7446-19-7 In Bulk Supply
- Molecular Formula:H2O5SZn
- Molecular Weight:179.49
- Appearance/Colour:white powder or granules
- Vapor Pressure:3.35E-05mmHg at 25°C
- Melting Point:decomposes at 238℃ [KIR84]
- Boiling Point:330 °C at 760 mmHg
- PSA:97.87000
- Density:1.005 g/mL at 25°C
- LogP:-0.32400
Zinc sulfate monohydrate(Cas 7446-19-7) Usage
|
Physical Properties |
The anhydrous sulfate is a colorless rhombohedral crystalline solid; refractive index 1.658; density 3.54 g/cm3; decomposes at 600°C; soluble in water, methanol, and glycerol. The heptahydrate, ZnSO4?7H2O, is a colorless crystalline solid; metallic taste; rhombohedral crystals; effloresces; refractive index 1.457; density 1.957 g/cm3 at 25°C; melts at 100°C; loses all its water molecules at 280°C; decomposes above 500°C; very soluble in water, 96.5 g/100mL at 20°C; soluble in glycerol, 40 g/100 mL; insoluble in alcohol. The hexahydrate, ZnSO4?6H2O constitutes colorless monoclinic or tetragonal crystals; density 2.072 g/cm3 at 15°C; loses five water molecules at 70°C; soluble in water. |
|
Occurrence and Uses |
Zinc sulfate occurs in nature as the mineral, zinkosite. The heptahydrate, ZnSO4?7H2O is the mineral, goslarite. The salt is used as a mordant in calicoprinting, in making rayon, in preserving wood, in animal feeds, in electroplating, and in preparing many zinc compounds. |
|
Production |
Zinc sulfate is produced as an intermediate in recovering zinc from mineral zinc blende, ZnS (see Zinc, Recovery). The mineral is roasted at about 1,000°C to form zinc oxide and sulfur dioxide which, on prolonged heating in excess air, converts to zinc sulfate: 2ZnS + 3O2 → 2ZnO + 2SO2 2ZnO + 2SO2 + O2 → 2ZnSO4 In the zinc recovery process, roasted products are leached with sulfuric acid, whereupon zinc oxide is converted to sulfate. ZnO + H2SO4 → ZnSO4 + H2O Also, zinc sulfate can be prepared by reacting metallic zinc with dilute sulfuric acid followed by evaporation and crystallization: Zn + H2SO4 → ZnSO4 + H2 |
|
Chemical Properties |
White, free-flowing powder. Soluble in water; insoluble in alcohol. |
|
Uses |
Zinc Sulfate Monohydrate is a special compound fertilizer for improving rapeseed yield. |
|
Purification Methods |
Crystallise it from aqueous EtOH or dilute H2SO4 below 39o when it forms the heptahydrate, and between 39o and 70o it forms the hexahydrate, and above 70o the monohydrate is stable. The anhydrous salt is obtained from the hydrates by heating at 280o or lower temperatures in a current of dry air. It decomposes to ZnO and SO2 at 767o. The solubility of the heptahydrate in H2O is 5.88% at 0o, 61.92% at 30o, 66.61% at 35o and 70.05% at 39o. |
InChI:InChI=1/H2O4S.H2O.Zn/c1-5(2,3)4;;/h(H2,1,2,3,4);1H2;/q;;+2/p-2
Relevant Products
-
SODIUM SULFIDE PENTAHYDRATE
CAS:1313-83-3
-
Copper(II) chloride dihydrate
CAS:10125-13-0
-
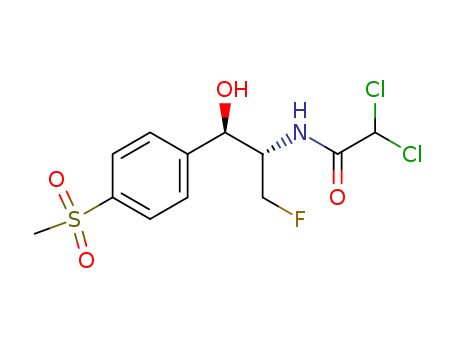
Florfenicol
CAS:73231-34-2