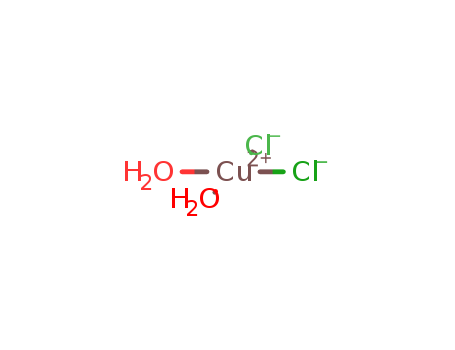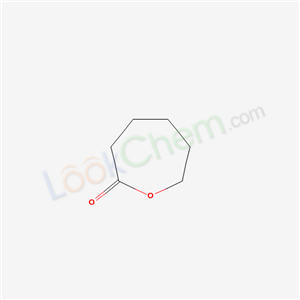
24980-41-4
- Product Name:Poly(ε-caprolactone)
- Molecular Formula:C6H10O2
- Purity:99%
- Molecular Weight:114.1424
Product Details;
CasNo: 24980-41-4
Molecular Formula: C6H10O2
Buy High Grade Poly(ε-caprolactone),Export 24980-41-4 Safe Shipping
- Molecular Formula:C6H10O2
- Molecular Weight:114.1424
- Vapor Pressure:0.0866mmHg at 25°C
- Melting Point:60 °C(lit.)
- Boiling Point:225.4°Cat760mmHg
- Flash Point:84.8°C
- PSA:26.30000
- Density:1.021g/cm3
- LogP:1.10360
Poly(ε-caprolactone)(Cas 24980-41-4) Usage
|
Description |
Poly(ε-caprolactone) (PCL) is a synthetic, semi-crystalline, and biodegradable polyester with versatile applications. Known for its biocompatibility, bioresorbability, and FDA and CE approval as a medical device, PCL is a chemically synthesized material with repeating units containing non-polar methylene-CH2 starch. It can be blended with other substances to create completely biodegradable materials. |
|
Chemical Properties |
intrinsic viscosity 1.00-1.30 |
|
Uses |
Primarily used in biomedical and environmental applications, PCL is employed in research medical devices and tissue engineering solutions, particularly for orthopedic or soft tissue fixation devices. The material's controlled degradation, achieved through modification of molecular weight and polymer composition, ensures safe resorption by the body after implantation. PCL's slow degradation rate, attributed to its aliphatic chain length, distinguishes it from other biodegradable polymers like polylactide. Its low melting point and high solubility in organic solvents make it ideal for thermal processing and various applications, including 3D bioprinting. With low residual water, monomer, and catalyst content, PCL stands out as a preferred choice for tissue engineering and 3D bioprinting research. |
InChI:InChI=1/C6H10O2/c7-6-4-2-1-3-5-8-6/h1-5H2
24980-41-4 Relevant articles
Poly-ϵ-caprolactone microspheres and nanospheres: an overview
V.R. Sinha, K. Bansal, R. Kaushik, R. Kumria, A. Trehan
, International Journal of Pharmaceutics Volume 278, Issue 1, 18 June 2004, Pages 1-23
Most of the research work has been directed at poly(lactic acid), poly(glycolic acid), poly(lactic-co-glycolic acid) (PLGA) copolymers. The success of these for pharmaceutical applications has further led to the evaluation of aliphatic polyesters such as poly-ε-caprolactone (PCL).
A novel method for epoxidation of cyclohexene catalyzed by Fe2O3 with molecular oxygen and aldehydes
Li, Xuegeng,Wang, Fan,Lu, Xiaoling,Song, Guoqiang,Zhang, Hao
, p. 2075 - 2079 (1997)
A novel method for the epoxidation of cy...
Synthesis and Degradation Behavior of Cyclic Poly(ε-caprolactone)
Jessica N. Hoskins and Scott M. Grayson*
, Macromolecules 2009, 42, 17, 6406–6413
Narrow polydisersity cyclic poly(caprolactone) was synthesized by cyclization of linear α,ω-functionalized poly(caprolactone). The linear precursors were prepared via ring-opening polymerization from an azido-functionalized initiator, followed by end group modification to attach a terminal alkyne. Click coupling afforded the cyclic polymer in high yields and provided linear and cyclic poly(caprolactone) with exactly identical molecular weight distributions. The thermal and acid-catalyzed degradation of analogous linear and cyclic poly(caprolactone) samples were investigated to determine the effect of architecture.
Lipase catalysed oxidations in a sugar-derived natural deep eutectic solvent
Vagnoni, Martina,Samorì, Chiara,Pirini, Daniele,Vasquez De Paz, Maria Katrina,Gidey, Dawit Gebremichael,Galletti, Paola
, (2021/05/06)
Chemoenzymatic oxidations involving the ...
24980-41-4 Process route
-
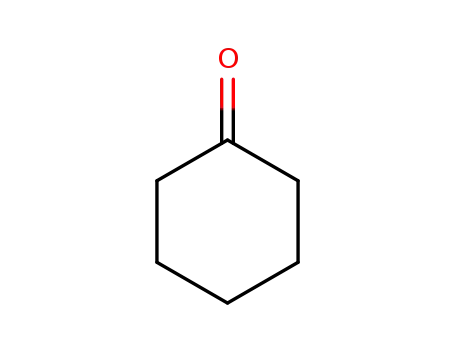
- 108-94-1,11119-77-0,9003-41-2,9075-99-4
cyclohexanone

-
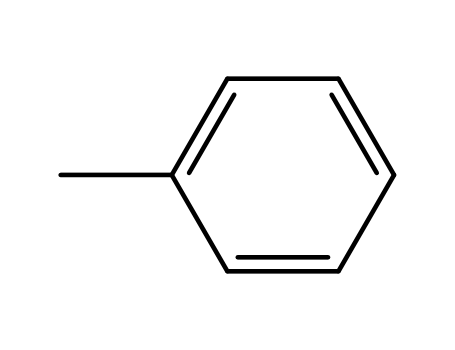
- 108-88-3,15644-74-3,16713-13-6
toluene

-
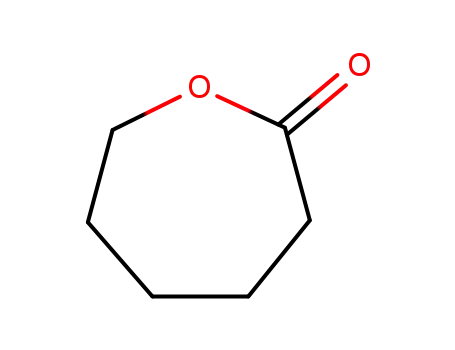
- 502-44-3,24980-41-4,80137-66-2
hexahydro-2H-oxepin-2-one

-
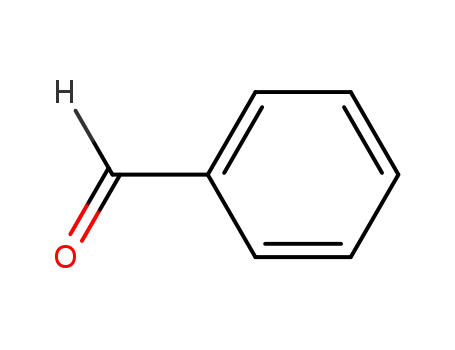
- 100-52-7
benzaldehyde

-
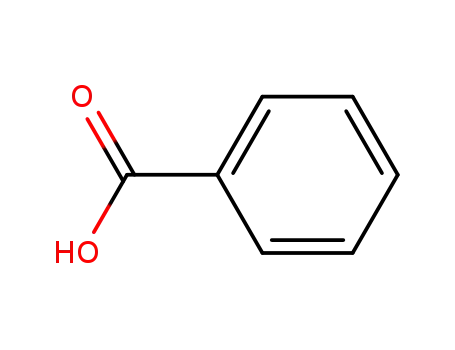
- 65-85-0,8013-63-6
benzoic acid
| Conditions | Yield |
|---|---|
|
With Iron(III) nitrate nonahydrate; N-hydroxyphthalimide; In acetonitrile; at 40 ℃; for 10h; under 1500.15 Torr; Temperature; Solvent; Large scale;
|
0.464 kg 1.76 kg 0.403 kg |
-
![7,15,16-Trioxa-dispiro[5.1.6.2]hexadecane](/upload/2024/1/a3baa573-7f37-437a-875d-a91bf68b1104.png)
- 185614-71-5
7,15,16-Trioxa-dispiro[5.1.6.2]hexadecane

-

- 502-44-3,24980-41-4,80137-66-2
hexahydro-2H-oxepin-2-one

-
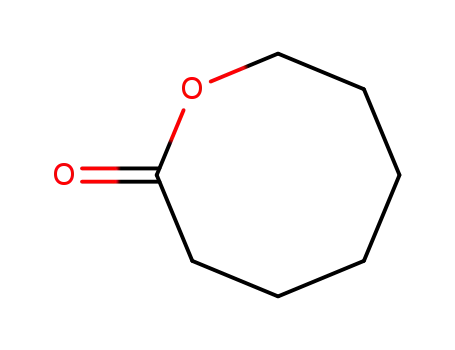
- 539-87-7
oxocan-2-one

-

- 108-94-1,11119-77-0,9003-41-2,9075-99-4
cyclohexanone

-
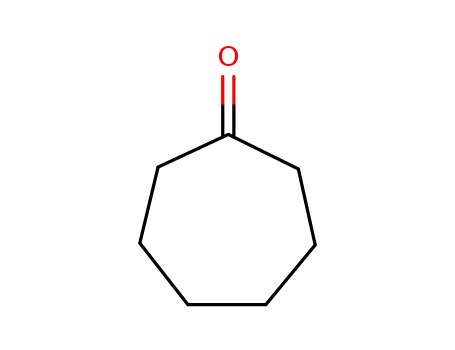
- 502-42-1
cycloheptanone
| Conditions | Yield |
|---|---|
|
With titanium tetrachloride; at -78 ℃; Further Variations:; Reagents; Temperatures; Product distribution;
|
43% 5% 11% 41% |
24980-41-4 Upstream products
-
109-99-9
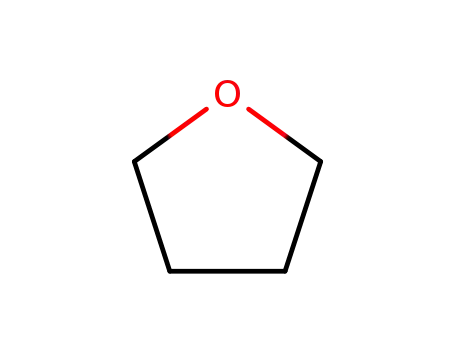
tetrahydrofuran
-
463-51-4
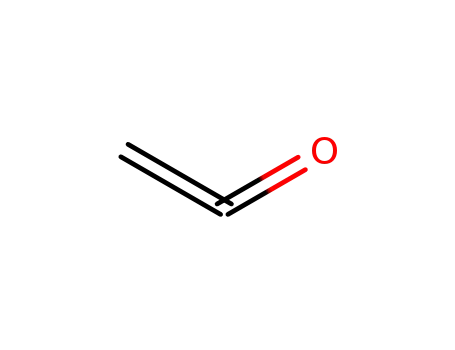
Ketene
-
1191-25-9
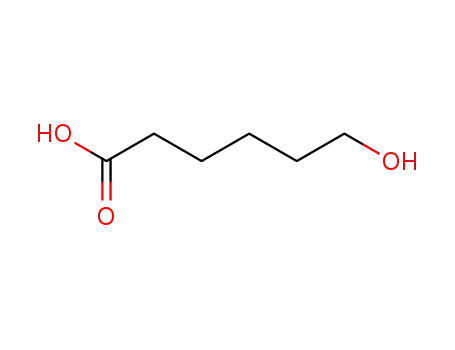
6-Hydroxyhexanoic acid
-
35784-01-1
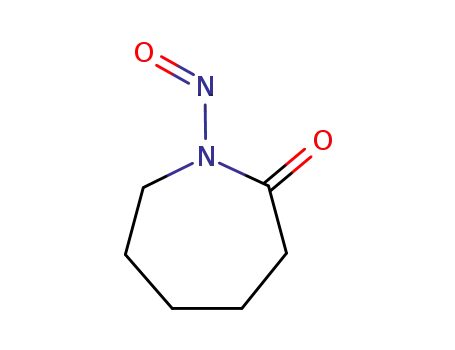
N-nitroso-6-caprolactam
24980-41-4 Downstream products
-
1577-22-6
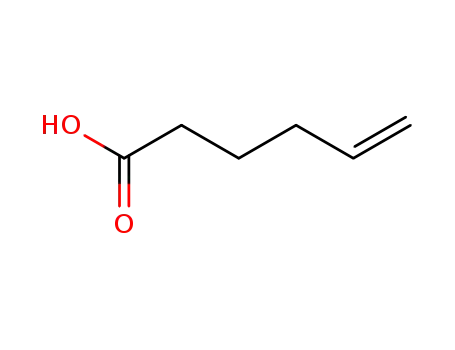
5-hexenoic acid
-
39979-08-3
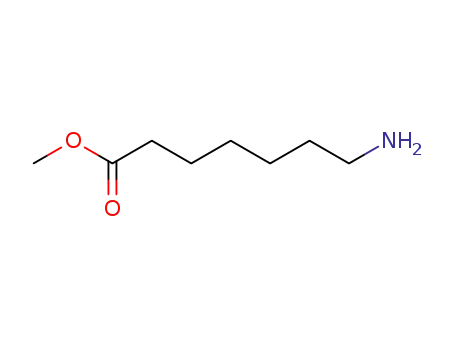
methyl 7-aminoheptanoate
-
14273-90-6
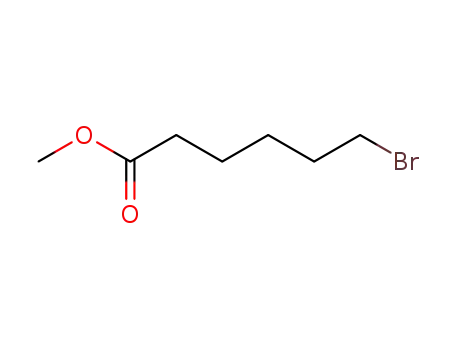
methyl 6-bromohexanoate
-
1117-66-4
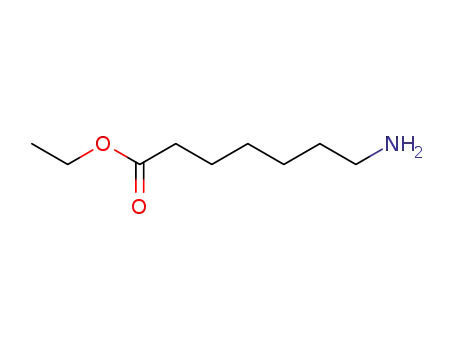
7-aminoheptanoic acid ethyl ester
Relevant Products
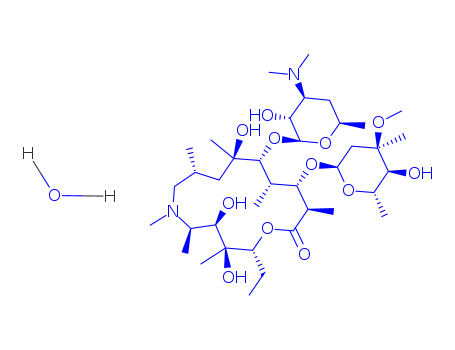
-
Azithromycin dihydrate
CAS:117772-70-0
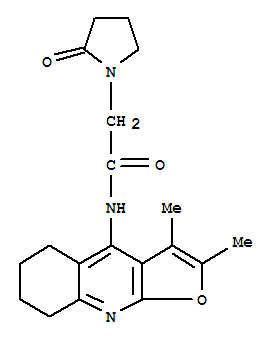
-
COLURACETAM
CAS:135463-81-9
-
Copper(II) chloride dihydrate
CAS:10125-13-0