857890-39-2
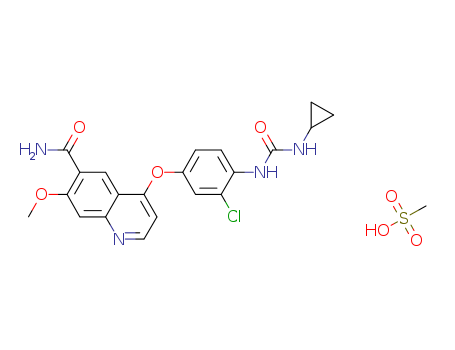
- Product Name:lenvatinib Mesylate
- Molecular Formula:C21H19ClN4O4.CH4O3S
- Purity:99%
- Molecular Weight:522.966
Product Details;
CasNo: 857890-39-2
Molecular Formula: C21H19ClN4O4.CH4O3S
Buy High Quality Best Quality lenvatinib Mesylate 857890-39-2 In Bulk Supply
- Molecular Formula:C21H19ClN4O4.CH4O3S
- Molecular Weight:522.966
- PSA:178.32000
- LogP:5.82090
857890-39-2 Relevant articles
Novel method for the synthesis of lenvatinib using 4-nitrophenyl cyclopropylcarbamate and their pharmaceutical salts
Sadineni, Ravi Kumar,Rapolu, Rajesh Kumar,Raju, V. V. N. K. V. Prasada,Srinivasu,Malladi, Sireesha,Mulakayala, Naveen
, p. 1475 - 1483 (2020/11/05)
4-Nitrophenyl cyclopropylcarbamate was d...
Synthesis method of lenvatinib and new intermediate
-
Paragraph 0049; 0062-0064, (2020/07/24)
The invention discloses a synthesis meth...
Preparation method of high-purity lenvatinib mesylate crystal form C
-
Paragraph 0054-0072, (2020/10/04)
The invention belongs to the technical f...
Method for refining Lenvatinib mesylate
-
Paragraph 0033-0039, (2020/03/02)
The invention discloses a method for ref...
857890-39-2 Process route
-
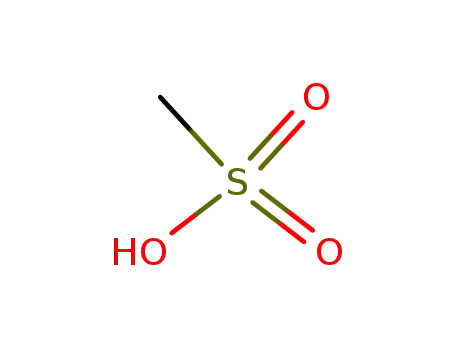
- 75-75-2,98527-29-8
methanesulfonic acid

-
![1-{2-chloro-4-[(6-cyano-7-methoxy-quinolin-4-yl)oxy]phenyl}-3-cyclopropylurea](/upload/2024/1/2389eda0-2021-4d50-b689-a2fc4d24892d.png)
- 1882873-21-3
1-{2-chloro-4-[(6-cyano-7-methoxy-quinolin-4-yl)oxy]phenyl}-3-cyclopropylurea

-
![4-[3-chloro-4-(cyclopropylaminocarbonyl)aminophenoxy]-7-methoxy-6-quinoline-carboxamide methanesulfonate](/upload/2024/1/3fc57694-9ccf-45aa-8a04-2e83d8e02790.png)
- 857890-39-2
4-[3-chloro-4-(cyclopropylaminocarbonyl)aminophenoxy]-7-methoxy-6-quinoline-carboxamide methanesulfonate
| Conditions | Yield |
|---|---|
|
In ethanol; water; at 20 ℃; Solvent; Temperature; Industrial scale;
|
92% |
-
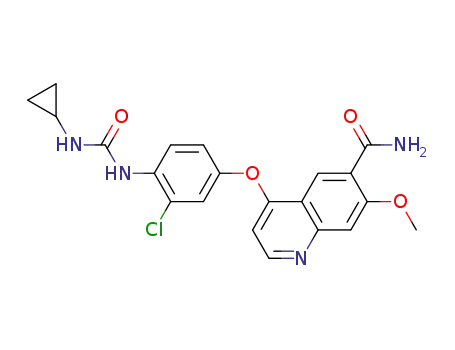
- 417716-92-8
lenvatinib

-

- 75-75-2,98527-29-8
methanesulfonic acid

-
![4-[3-chloro-4-(cyclopropylaminocarbonyl)aminophenoxy]-7-methoxy-6-quinoline-carboxamide methanesulfonate](/upload/2024/1/3fc57694-9ccf-45aa-8a04-2e83d8e02790.png)
- 857890-39-2
4-[3-chloro-4-(cyclopropylaminocarbonyl)aminophenoxy]-7-methoxy-6-quinoline-carboxamide methanesulfonate
| Conditions | Yield |
|---|---|
|
In tetrahydrofuran; water; at 20 ℃; for 3.66667h;
|
99% |
|
In ethanol; at 15 - 25 ℃; for 3h; Solvent; Time;
|
95.8% |
|
With acetic acid; at 20 - 35 ℃;
|
94% |
|
In methanol; at 20 ℃;
|
92.9% |
|
In N,N-dimethyl acetamide; at 25 - 65 ℃;
|
86.5% |
|
In isopropyl alcohol; at 80 - 85 ℃;
|
82% |
|
In methanol; at 20 - 75 ℃; for 22h; Inert atmosphere;
|
74.5% |
|
In methanol; at 20 - 70 ℃; for 24h; Product distribution / selectivity;
|
|
|
lenvatinib; methanesulfonic acid; In acetic acid; isopropyl alcohol; at 40 ℃; for 0.333333h;
In Isopropyl acetate; acetic acid; isopropyl alcohol; at 15 - 40 ℃; for 16h; Product distribution / selectivity;
|
|
|
lenvatinib; methanesulfonic acid; In acetic acid; at 20 - 70 ℃; for 24h;
In ethanol; acetic acid; at 20 - 40 ℃; for 12h; Product distribution / selectivity;
|
|
|
In acetic acid; isopropyl alcohol; at 17.6 - 30 ℃; for 1.4h; Product distribution / selectivity;
|
|
|
In methanol; at 20 - 70 ℃; for 14h; Product distribution / selectivity;
|
|
|
With acetic acid; In ethyl acetate; at 20 - 50 ℃; for 10h;
|
|
|
With acetic acid; In propan-1-ol; Isopropyl acetate; at 25 - 40 ℃; for 2.66667h;
|
|
|
With acetic acid; In dimethyl sulfoxide; ethyl acetate; at 20 - 80 ℃; for 20h; Product distribution / selectivity;
|
|
|
With acetic acid; In Isopropyl acetate; isopropyl alcohol; at 15 - 40 ℃; for 16.3333h; Product distribution / selectivity;
|
|
|
With acetic acid; In isopropyl alcohol; at 20 - 30 ℃; for 1.4h; Product distribution / selectivity;
|
|
|
With acetic acid; In ethanol; at 20 - 50 ℃; for 12h; Product distribution / selectivity;
|
|
|
lenvatinib; methanesulfonic acid; In acetic acid; isopropyl alcohol; at 40 ℃; for 0.333333h;
In Isopropyl acetate; acetic acid; isopropyl alcohol; at 15 ℃; for 16h; Product distribution / selectivity;
|
|
|
lenvatinib; methanesulfonic acid; In acetic acid; isopropyl alcohol; at 17.6 - 30 ℃; for 1.4h;
In ethanol; at 20 ℃; for 1h; Product distribution / selectivity;
|
|
|
In dimethyl sulfoxide; isopropyl alcohol; at 15 - 62 ℃; for 3 - 3.5h; Product distribution / selectivity;
|
|
|
In ethanol; acetic acid; at 20 - 50 ℃; for 12h; Product distribution / selectivity;
|
|
|
In methanol; at 20 - 70 ℃; for 24h; Product distribution / selectivity;
|
|
|
In isopropyl alcohol; at 60 - 65 ℃; for 5h;
|
1.1 g |
|
In acetonitrile; at 20 ℃; for 24h;
|
|
|
at 25 - 30 ℃; for 24h;
|
|
|
With acetic acid; at 20 - 30 ℃; for 0.5h;
|
10.3 g |
|
With acetic acid; In propan-1-ol; at 25 - 30 ℃; for 0.333333h; Solvent; Temperature; Reagent/catalyst;
|
1.8 g |
|
In isopropyl alcohol; at 40 ℃; for 1h; Temperature; Solvent;
|
1.12 g |
|
In acetonitrile; at 20 ℃; for 24h; Solvent; Temperature;
|
857890-39-2 Upstream products
-
417716-92-8

lenvatinib
-
75-75-2

methanesulfonic acid
-
417722-93-1
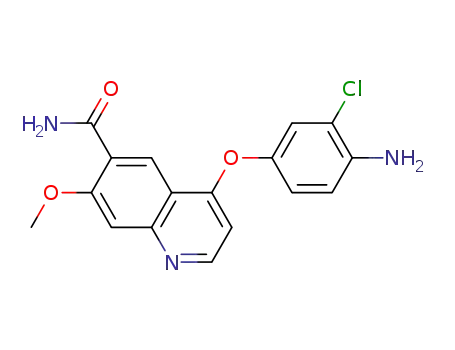
4?(4?amino?3?chlorophenoxy)?7?methoxyquinoline?6?carboxamide
-
52671-64-4
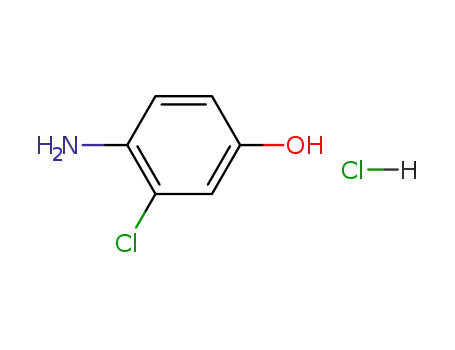
4-Amino-3-chlorophenol hydrochloride
Relevant Products
-
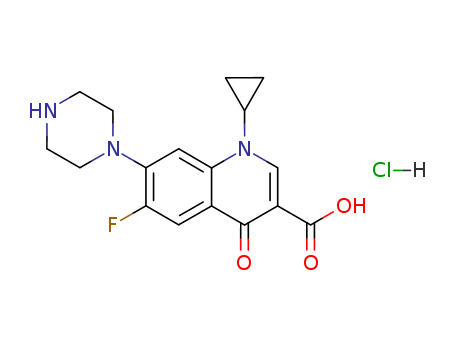
Ciprofloxacin HCl
CAS:93107-08-5
-
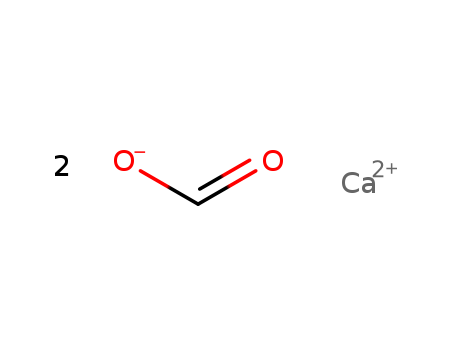
Calcium formate
CAS:544-17-2
-
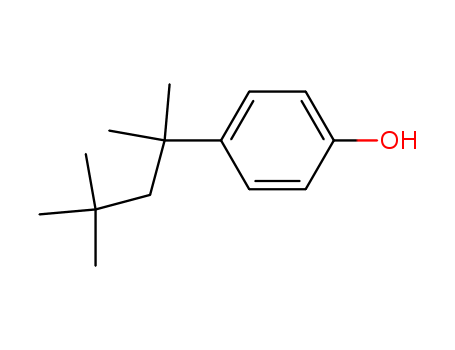
4-tert-Octylphenol
CAS:140-66-9