140-66-9
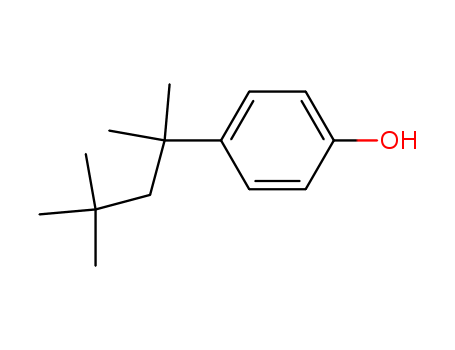
- Product Name:4-tert-Octylphenol
- Molecular Formula:C14H22O
- Purity:99%
- Molecular Weight:206.328
Product Details;
CasNo: 140-66-9
Molecular Formula: C14H22O
Appearance: white powder
Buy Reliable Quality High Purity 99% 4-tert-Octylphenol 140-66-9 Low Price
- Molecular Formula:C14H22O
- Molecular Weight:206.328
- Appearance/Colour:white powder
- Vapor Pressure:0.00025mmHg at 25°C
- Melting Point:79-82 °C(lit.)
- Refractive Index:1.507
- Boiling Point:282.3 °C at 760 mmHg
- PKA:10.15±0.15(Predicted)
- Flash Point:148.3 °C
- PSA:20.23000
- Density:0.935 g/cm3
- LogP:4.10600
4-tert-Octylphenol(Cas 140-66-9) Usage
|
Chemical Properties |
white powder |
|
Uses |
Despite its health risks, 4-tert-Octylphenol is used in manufacturing processes as an intermediate for surfactants, synthetic rubber additives, and resins. Additionally, it serves in the production of alkylphenol ethoxylates. Commonly found in detergents, cleaning products, and emulsifiers, it is also present, though less frequently, in paints, personal care products, pesticides, paper, pulp, and textiles. The widespread use of 4t-OP highlights the need for awareness and regulation due to its potential adverse effects on both human health and the environment. |
|
General Description |
4-tert-Octylphenol (4t-OP) is a chemical known for its toxicity and estrogenic properties in mammalian cells, making it an endocrine-disrupting chemical with potential harm to human health. It acts as a weak estrogenic pollutant, binding to estrogen receptors and exhibiting estrogenic effects in vitro. Studies have investigated its transformation under irradiation and hydroxyl radical exposure. This substance has been identified as detrimental to the male reproductive system in vertebrates, causing alterations in sex hormone levels, estrus cycles, reproductive outcomes, neonatal sexual development, and leading to impaired steroid hormone production and testicular atrophy at high doses. |
|
Environmental Fate |
Studies have shown that 4t-OP will adsorb to sediments. |
|
Purification Methods |
Crystallise the phenol from n-hexane and/or distil it in a vacuum. [Beilstein 6 III 2051, 6 IV 3484.] |
|
Toxicity evaluation |
4t-OP has been shown to be an estrogen receptor (ER) agonist. Estrogenic effects of 4t-OP have been demonstrated in human cells, with 4t-OP displacing the natural estrogen 17b estradiol from its receptor, and 4t-OP inducing cell proliferation in estrogen-dependent cell proliferation assays. In vivo confirmation of 4t-OP’s estrogenicity has been confirmed with the uterotrophic assay. |
InChI:InChI=1/C14H22O/c1-2-3-4-5-6-7-8-13-9-11-14(15)12-10-13/h9-12,15H,2-8H2,1H3
140-66-9 Relevant articles
4-Nonylphenols and 4-tert-octylphenol in water and fish from rivers flowing into Lake Biwa
Taizo Tsuda, Akihiko Takino, Mihoko Kojima, Hiroyuki Harada, Kazue Muraki, Motohiro Tsuji
, Chemosphere Volume 41, Issue 5, September 2000, Pages 757-762
Surveys of 4-nonylphenols (NOs) and 4-tert-octylphenol (OC) were performed for water and fish samples obtained from eight rivers flowing into Lake Biwa once every two months from April 1998 to March 1999. For water samples, NOs were detected all the year round (0.11–3.08 ng ml−1) at high frequency (48/48) in the eight rivers.
Alkylation of Phenol with tert-Butanol in a Draining-Film Reactor
Maksimov, A. L.,Mel’chakov, I. S.,Terekhov, A. V.,Zanaveskin, L. N.
, p. 569 - 575 (2021/07/26)
The alkylation of phenol with tert-butan...
Chloroindate(iii) ionic liquids as catalysts for alkylation of phenols and catechol with alkenes
Gunaratne, H. Q. Nimal,Lotz, Tobias J.,Seddon, Kenneth R.
scheme or table, p. 1821 - 1824 (2011/01/07)
Chloroindate(iii) ionic liquids are show...
Synthesis of 4-tert-octylphenol and 4-cumylphenol by metal triflate and metal triflimidate catalysts
Le Rouzo, Guillaume,Morel-Grepet, Marielle,Simonato, Jean-Pierre
, p. 521 - 522 (2007/10/03)
Metal inflates and metal triflimidates a...
140-66-9 Process route
-
- 75-65-0
tert-butyl alcohol

-
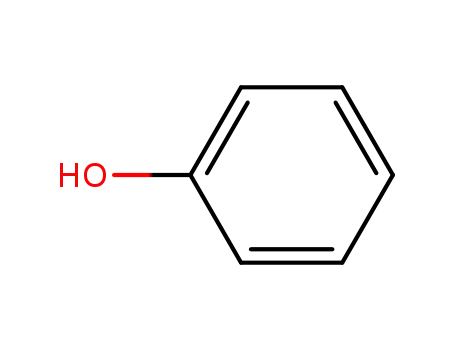
- 108-95-2,27073-41-2
phenol

-
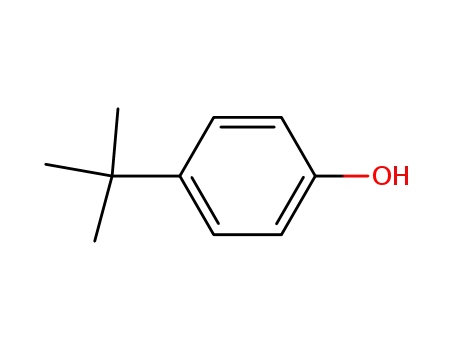
- 98-54-4
para-tert-butylphenol

-
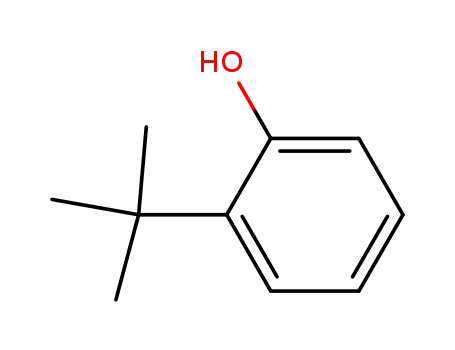
- 88-18-6
2-tert-Butylphenol

-
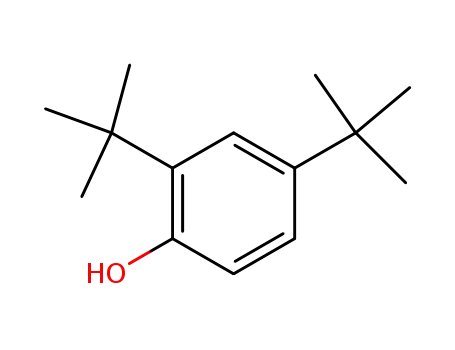
- 96-76-4
2,4-di-tert-Butylphenol

-
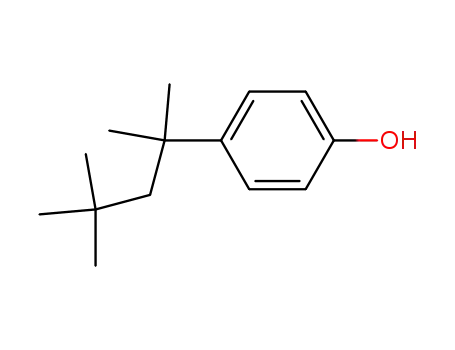
- 140-66-9
tert-octylphenol

-
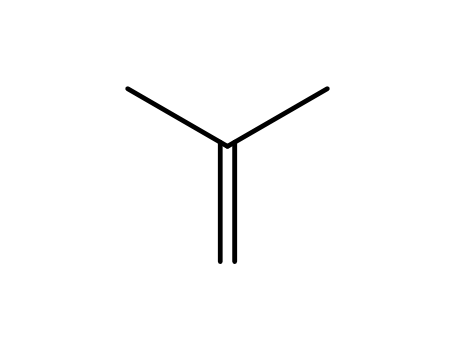
- 115-11-7,15220-85-6
isobutene
| Conditions | Yield |
|---|---|
|
With beta zeolite; at 90 ℃; Temperature;
|
-
![4-[4-(1,1,3,3-Tetramethyl-butyl)-phenoxycarbonylmethyl]-benzoic acid](/upload/2024/1/5cb57c1c-f34d-4c29-a7d8-3adbc5bc54a3.png)
-
4-[4-(1,1,3,3-Tetramethyl-butyl)-phenoxycarbonylmethyl]-benzoic acid

-
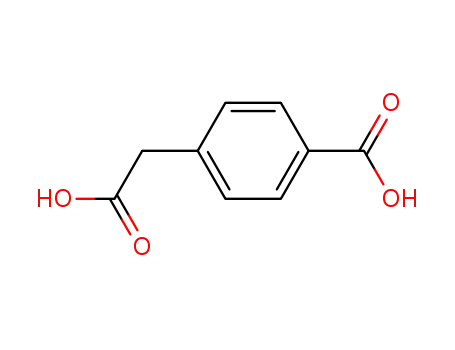
- 501-89-3
4-(carboxymethyl)benzoic acid

-

- 140-66-9
tert-octylphenol
| Conditions | Yield |
|---|---|
|
With phosphate buffer; In acetonitrile; at 21 ℃; Rate constant; other buffer; different pH; also in the presence of amidine or molecularly imprinted polymers as catalysts;
|
140-66-9 Upstream products
-
872823-89-7
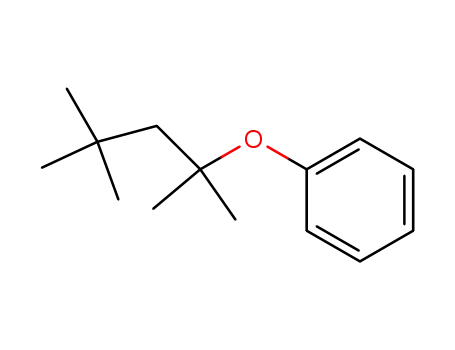
phenyl-(1,1,3,3-tetramethyl-butyl)-ether
-
42265-56-5
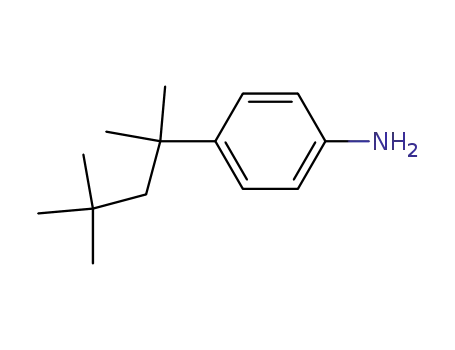
4,4-di-tert-octylphenylamine
-
107-39-1
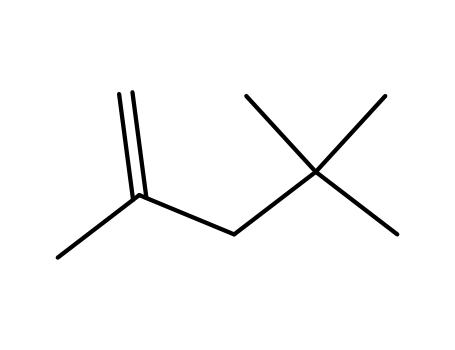
2,4,4-trimethyl-1-pentene
-
108-95-2

phenol
140-66-9 Downstream products
-
2315-67-5
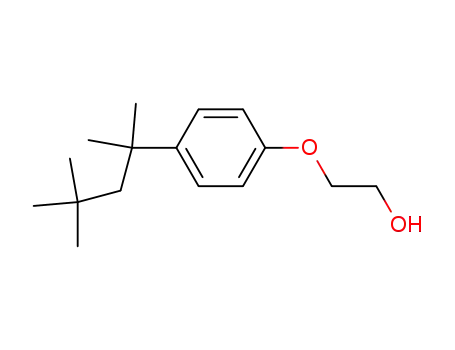
2-[4-(2,4,4-trimethylpentan-2-yl)phenoxy]ethanol
-
5414-83-5
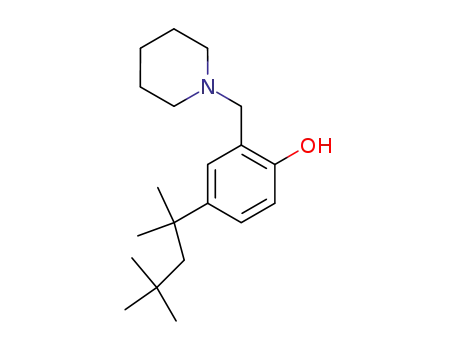
2-(piperidin-1-ylmethyl)-4-(2,4,4-trimethylpentan-2-yl)phenol
-
25543-98-0
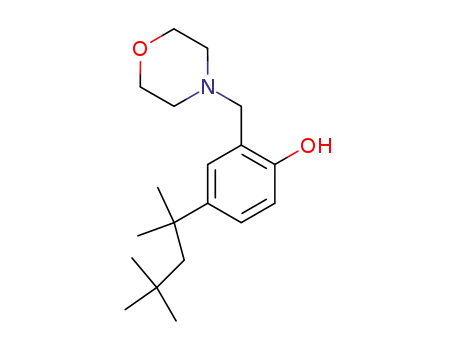
2-Morpholinomethyl-4-(1,1,3,3-tetramethyl-butyl)-phenol
-
5454-15-9
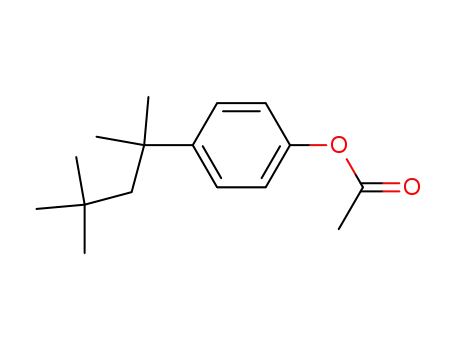
1-acetoxy-4-(1,1,3,3-tetramethyl-butyl)-benzene
Relevant Products
-
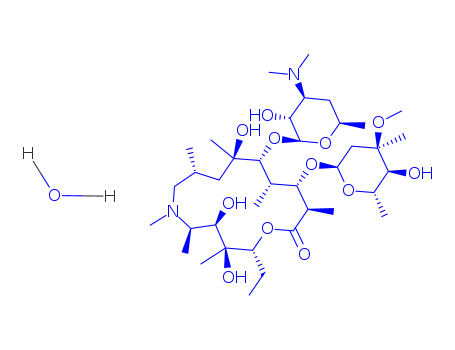
Azithromycin dihydrate
CAS:117772-70-0
-
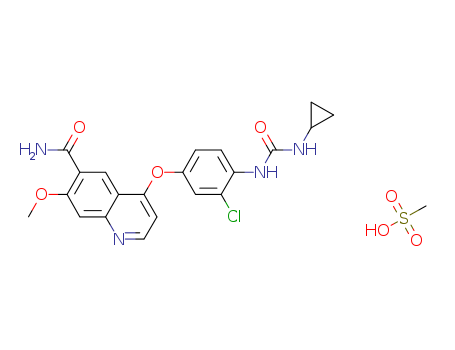
lenvatinib Mesylate
CAS:857890-39-2
-
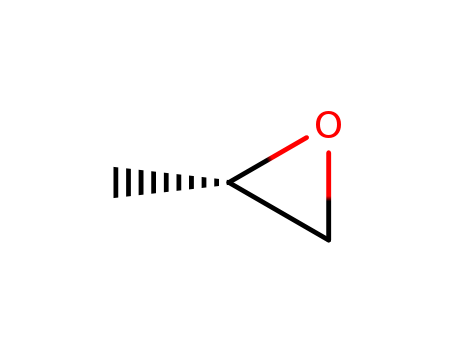
(S)-(-)-Propylene oxide
CAS:16088-62-3