544-17-2
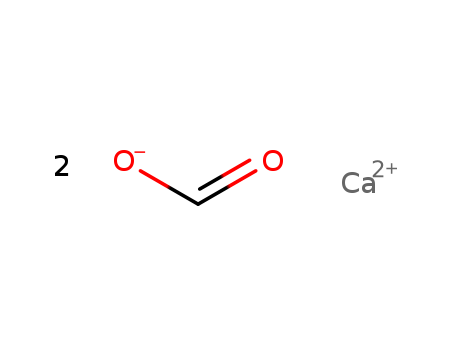
- Product Name:Calcium formate
- Molecular Formula:C2H2CaO4
- Purity:99%
- Molecular Weight:130.113
Product Details;
CasNo: 544-17-2
Molecular Formula: C2H2CaO4
Appearance: white to almost white fine crystalline powder
Factory Supply High Purity Calcium formate 544-17-2,Buy 544-17-2 Efficient Delivery
- Molecular Formula:C2H2CaO4
- Molecular Weight:130.113
- Appearance/Colour:white to almost white fine crystalline powder
- Vapor Pressure:3.41Pa at 25℃
- Melting Point:300 °C
- Boiling Point:100.6 °C at 760 mmHg
- PKA:3.8[at 20 ℃]
- Flash Point:29.9 °C
- PSA:52.60000
- Density:2.02 g/cm3
- LogP:0.51920
Calcium formate(Cas 544-17-2) Usage
|
Description |
Calcium formate is a white to almost white fine crystalline powder. It can be used an accelerator for the pozzolanic cement pastes. On the one hand, it shortens the initial and final setting times and increases the compressive strength and combined water content as well as gel/space ratio at all ages of hydration. On the other hand, it decreases the total porosity. It has been shown that it has a growth-promoting effect in weanling pigs challenged with E. coli, independently of their susceptibility to the intestinal adhesion of this strain. More importantly, calcium formate can be used as a nutrient supplement to the feed of young growing pigs or fattening poultry, further boosting the growth of animals and the feed utilization. At the same time, it causes a reduction of the occurrence of piglet diarrhea. |
|
Chemical Properties |
Calcium formate, Ca(HCOO)2, is the calcium salt of formic acid, HCOOH. It is also known as food additive E238 in food industry. The mineral form is very rare and called formicaite. It is known from a few boron deposits. It may be produced synthetically by reacting calcium oxide or calcium hydroxide with formic acid. |
|
Uses |
Calcium Formate is Hydrophobic additives for construction materials.Formic Acid is used as a potential energy source in the preparation of fuel cells. Also used in chemical synthesis of various anti-inflammatory and anti-microbial agents. |
|
Reactions |
Calcium formate can be used to prepare solutions of other water-soluble formate salts. For example, the addition of nickel (II) sulfate to a solution of calcium formate results in the precipitation of insoluble calcium sulfate, leaving nickel (II) formate in solution: Ca(OOCH)2 + NiSO1 → CaSO4 + Ni(OOCH)2 In a similar reaction, sulfuric acid reacts with calcium formate to provide low-cost solutions of formic acid: Ca(OOCH)2 + H2 SO4 → CaSO4 + 2HCOOH When heated, calcium formate decomposes to calcium carbonate and formaldehyde: Ca(OOCH)2 → CaCO3 + HCHO Calcium formate can be used as a low-cost reducing agent in the conversion of carboxylic acids to aldehydes. This process is an effective substitute for the troublesome Rosenmund reduction. (RCOO)2Ca + (HCOO)2CA → 2RCH + 2CaCO3 |
|
Flammability and Explosibility |
Notclassified |
|
Safety Profile |
Poison by intravenous route. Moderately toxic by ingestion. An eye irritant. When heated to decomposition it emits acrid smoke and fumes. See also CALCIUM COMPOUNDS. |
|
Purification Methods |
Recrystallise it from water (5mL/g) by partial evaporation in a desiccator. [Beilstein 2 IV 16.] |
|
Toxicity evaluation |
Calcium formate has a low order of toxicity. In rats, the acute oral LD50 is 2650mg/kg; by intravenous injection, the LD50 is 154mg/kg. In a study with rats, calcium formate added to the drinking water at 0.2% for 3 years or 0.4% for 2 years didn't affect growth, fertility or function in up to 5 generations. Calcium formate would be expected to be irritating to the eyes and skin. In case of eye contact, immediately flush the eyes with water for 15 minutes. Avoid prolonged or repeated exposure of the skin. |
InChI:InChI=1/CH2O2.Ca/c2-1-3;/h1H,(H,2,3);/q;+2/p-1
544-17-2 Relevant articles
Hydrogenation of aqueous mixtures of calcium carbonate and carbon dioxide using a water-soluble rhodium(I)-tertiary phosphine complex catalyst
Jószai, István,Joó, Ferenc
, p. 87 - 91 (2004)
Aqueous suspensions of calcium carbonate...
-
Balcerowiak, W.,Terelak, K.,Trybula, S.
, p. 1231 - 1238 (1995)
-
Eco-friendly upconversion of limestone into value-added calcium formate
Gunasekar, Gunniya Hariyanandam,Padmanaban, Sudakar,Park, Hongjin,Yoon, Sungho
, p. 4995 - 5001 (2020)
Although CaCO3 is a naturally available,...
Eco-friendly and techno-economic conversion of CO2 into calcium formate, a valuable resource
Lee, Chul-Jin,Yoon, Ha-Jun,Yoon, Hayoung,Yoon, Sungho,Yoon, Taeksang
, p. 1738 - 1745 (2022/03/08)
The suppression of greenhouse gas emissi...
A a method of preparing calcium
-
Paragraph 0022-0023, (2017/07/07)
The invention discloses a preparation me...
CO2-"Neutral" hydrogen storage based on bicarbonates and formates
Boddien, Albert,Gaertner, Felix,Federsel, Christopher,Sponholz, Peter,Mellmann, Doerthe,Jackstell, Ralf,Junge, Henrik,Beller, Matthias
, p. 6411 - 6414 (2011/08/05)
Let the circle be unbroken! One rutheniu...
544-17-2 Process route
-
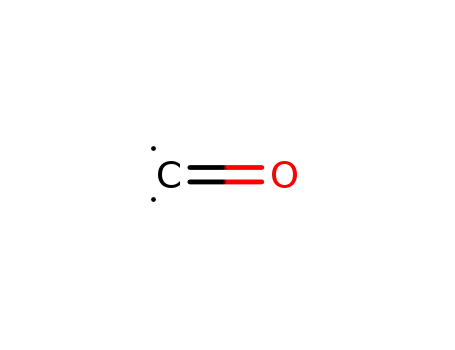
- 201230-82-2
carbon monoxide

-
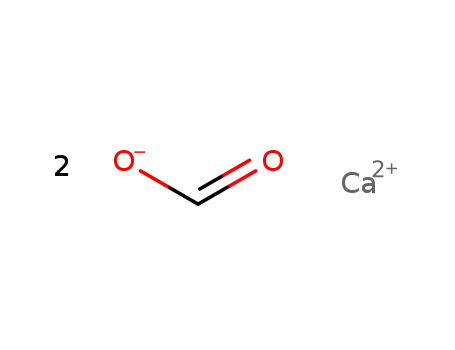
- 544-17-2
calcium diformate
| Conditions | Yield |
|---|---|
|
With calcium hydroxide; at 180 ℃; under 36775.4 Torr; Product distribution; temperatures from 100 to 200 deg C, pressure from 30 to 60 kg/cm2, effect of Ca(OH)2 concentration, optimal conditions for maximum yield achievement;
|
|
|
With methylamine; calcium hydroxide; at 165 - 170 ℃; Temperature; Reagent/catalyst;
|

-

- 544-17-2
calcium diformate
| Conditions | Yield |
|---|---|
|
With water; nickel; at 125 ℃; under 147102 Torr; Hydrogenation;
|
544-17-2 Upstream products
-
51437-68-4
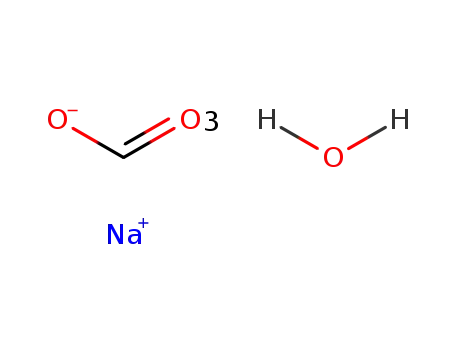
sodium formate *3H2O
-
201230-82-2
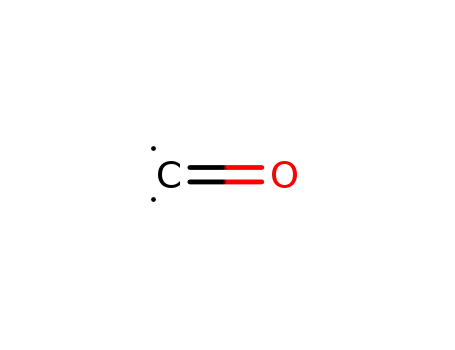
carbon monoxide
-
64-18-6
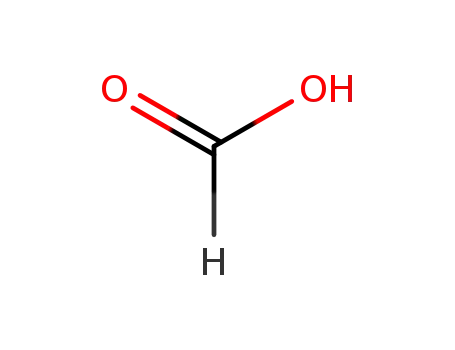
formic acid
-
124-38-9
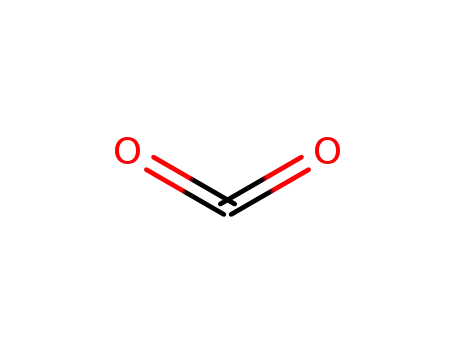
carbon dioxide
544-17-2 Downstream products
-
107-31-3
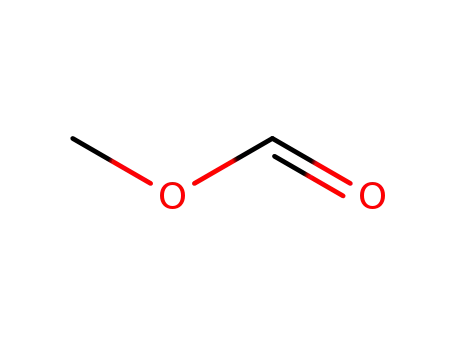
Methyl formate
-
64-18-6

formic acid
-
69-89-6
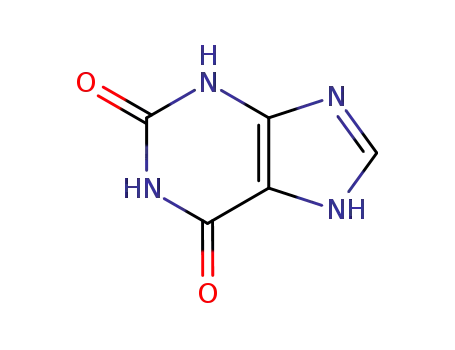
xanthin
-
104-55-2
3-phenyl-propenal
Relevant Products
-
Monosodium glutamate
CAS:32221-81-1
-
4-Cyano-4'-pentylbiphenyl
CAS:40817-08-1
-
lenvatinib Mesylate
CAS:857890-39-2