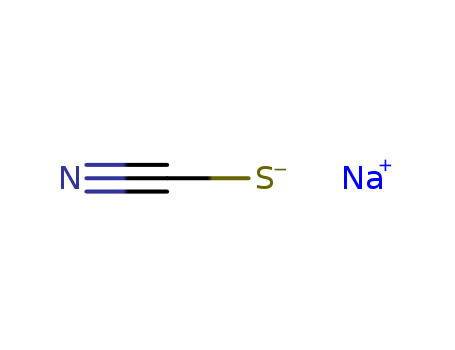
Product Details;
CasNo: 7440-44-0
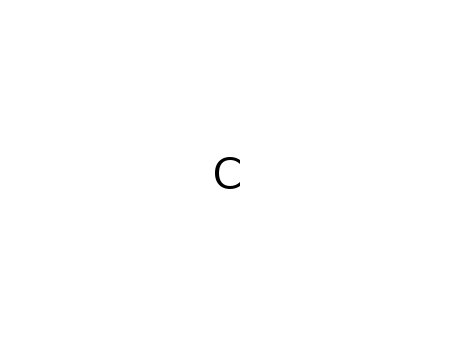
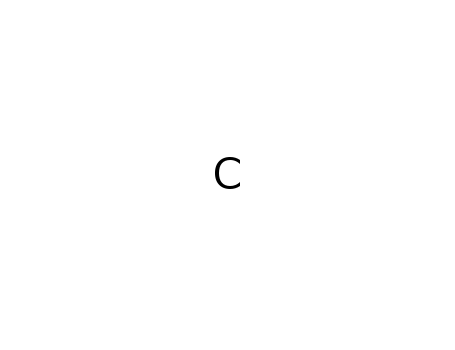
Molecular Formula: C
Appearance: grey solid
Chinese Factory Supply Top Purity 99% Carbon 7440-44-0 Efficient Transportation
- Molecular Formula:C
- Molecular Weight:12.011
- Appearance/Colour:grey solid
- Vapor Pressure:<0.1 mm Hg ( 20 °C)
- Melting Point:3550 °C(lit.)
- Boiling Point:500-600 °C(lit.)
- Flash Point:>230 °F
- PSA:0.00000
- Density:~1.7 g/mL at 25 °C(lit.)
- LogP:0.00000
- IDLH: N.D.See: IDLH INDEX
- IDLH:443
- IDLH:3805
Carbon(Cas 7440-44-0) Usage
|
Description |
All our SWNTs come packed as dry powders, which can be dispersed within the user's solvent of choice. |
|
Chemical Properties |
Carbon, C, is a nonmetallic element, grey solid. It is found in nature as graphite (specific gravity2.25), diamond(specific gravity 3.51), and coal (specific gravity 1.88). Carbon is found in all living things, is insoluble in common solvents,and forms an almost infinite numberof organic compounds. Anaturally occurring radioactive isotope,14C, has a half-life of 5780 years and is used in archaeo logical investigations to date artifacts and ancient documents. Other uses of carbon depend on its form. For example, diamonds for jewels and abrasives,graphite for lubricants, activated carbon to absorb color and gases, and wood carbon for fuel are some common examples. |
|
Physical properties |
All the elements in group 14 have four electrons in their outer valence shell. Carbon exhibitsmore nonmetallic properties than do the others in group 14 and is unique in several ways.It has four forms, called allotropes:1. Carbon black is the amorphous allotrope (noncrystal form) of carbon. It is produced byheating coal at high temperatures (producing coke); burning natural gas (producing jetblack); or burning vegetable or animal matter (such as wood and bone), at high temperatureswith insufficient oxygen, which prevents complete combustion of the material, thusproducing charcoal.2. Graphite is a unique crystal structure of carbon wherein layers of carbon atoms are stackedparallel to each other and can extend indefinitely in two dimensions as in the shafts ofcarbon fiber golf clubs. Graphite is also one of the softest elements, making it an excellentdry lubricant.3. Diamonds are another allotrope whose crystal structure is similar to graphite. Naturaldiamonds were formed under higher pressure and extreme temperatures. Synthetic diamondshave been artificially produced since 1955.4. Fullerenes are another amorphous (no crystal structure) form of carbon that have the basicformula of C60H60 and are shaped like a soccer ball. (See the “Atomic Structure” sectionof the book for more on fullerenes.)The different allotropes of carbon were formed under varying conditions in the Earth,starting with different minerals, temperature, pressure, and periods of time. Once the distinctcrystal structures are formed, they are nearly impossible to change.Carbon-12 is the basis for the average atomic mass units (amu) that is used to determinethe atomic weights of the elements. Carbon is one of the few elements that can form covalentbonds with itself as well as with many metals and nonmetals. |
|
Isotopes |
There are 15 isotopes of carbon, two of which are stable. Stable carbon-12makes up 98.89% of the element’s natural abundance in the Earth’s crust, and carbon-13 makes up just 1.11% of carbon’s abundance in the Earth’s crust. All the otherisotopes of carbon are radioactive with half-lives varying from 30 nanoseconds (C-21) to5,730 years (C-14). |
|
Origin of Name |
Carbon’s name is derived from the Latin word carbo, which means, “charcoal.” |
|
Occurrence |
Carbon is the 14th most abundant element, making up about 0.048% of the Earth’s crust.It is the sixth most abundant element in the universe, which contains 3.5 atoms of carbonfor every atom of silicon. Carbon is a product of the cosmic nuclear process called fusion,through which helium nuclei are “burned” and fused together to form carbon atoms withthe atomic number 12. Only five elements are more abundant in the universe than carbon:hydrogen, helium, oxygen, neon, and nitrogen. |
|
Characteristics |
Carbon is, without a doubt, one of the most important elements on Earth. It is the majorelement found in over one million organic compounds and is the minor component in mineralssuch as carbonates of magnesium and calcium (e.g., limestone, marble, and dolomite),coral, and shells of oysters and clams.The carbon cycle, one of the most essential of all biological processes, involves the chemicalconversion of carbon dioxide to carbohydrates in green plants by photosynthesis. Animalsconsume the carbohydrates and, through the metabolic process, reconvert the carbohydratesback into carbon dioxide, which is returned to the atmosphere to continue the cycle. |
|
History |
Carbon, an element of prehistoric discovery, is very widely distributed in nature. It is found in abundance in the sun, stars, comets, and atmospheres of most planets. Carbon in the form of microscopic diamonds is found in some meteorites. Natural diamonds are found in kimberlite or lamporite of ancient formations called “pipes,” such as found in South Africa, Arkansas, and elsewhere. Diamonds are now also being recovered from the ocean floor off the Cape of Good Hope. About 30% of all industrial diamonds used in the U.S. are now made synthetically. The energy of the sun and stars can be attributed at least in part to the wellknown carbon-nitrogen cycle. Carbon is found free in nature in three allotropic forms: amorphous, graphite, and diamond. Graphite is one of the softest known materials while diamond is one of the hardest. Graphite exists in two forms: alpha and beta. These have identical physical properties, except for their crystal structure. Naturally occurring graphites are reported to contain as much as 30% of the rhombohedral (beta) form, whereas synthetic materials contain only the alpha form. The hexagonal alpha type can be converted to the beta by mechanical treatment, and the beta form reverts to the alpha on heating it above 1000°C. Of recent interest is the discovery of all-carbon molecules, known as “buckyballs” or fullerenes, which have a number of unusual properties. These interesting molecules, consisting of 60 or 70 carbon atoms linked together, seem capable of withstanding great pressure and trapping foreign atoms inside their network of carbon. They are said to be capable of magnetism and superconductivity and have potential as a nonlinear optical material. Buckyball films are reported to remain superconductive at temperatures as high as 45 K. In combination, carbon is found as carbon dioxide in the atmosphere of the Earth and dissolved in all natural waters. It is a component of great rock masses in the form of carbonates of calcium (limestone), magnesium, and iron. Coal, petroleum, and natural gas are chiefly hydrocarbons. Carbon is unique among the elements in the vast number and variety of compounds it can form. With hydrogen, oxygen, nitrogen, and other elements, it forms a very large number of compounds, carbon atom often being linked to carbon atom. There are close to ten million known carbon compounds, many thousands of which are vital to organic and life processes. Without carbon, the basis for life would be impossible. While it has been thought that silicon might take the place of carbon in forming a host of similar compounds, it is now not possible to form stable compounds with very long chains of silicon atoms. The atmosphere of Mars contains 96.2% CO2. Some of the most important compounds of carbon are carbon dioxide (CO2), carbon monoxide (CO), carbon disulfide (CS2), chloroform (CHCl3), carbon tetrachloride (CCl4), methane (CH4), ethylene (C2H4), acetylene (C2H2), benzene (C6H6), ethyl alcohol (C2H5OH), acetic acid (CH3COOH), and their derivatives. Carbon has fifteen isotopes. Natural carbon consists of 98.89% 12C and 1.11% 13C. In 1961 the International Union of Pure and Applied Chemistry adopted the isotope carbon-12 as the basis for atomic weights. Carbon-14, an isotope with a half-life of 5715 years, has been widely used to date such materials as wood, archeological specimens, etc. A new brittle form of car- 4-8 The Elements bon, known as “glassy carbon,” has been developed. It can be obtained with high purity. It has a high resistance to corrosion, has good thermal stability, and is structurally impermeable to both gases and liquids. It has a randomized structure, making it useful in ultra-high technology applications, such as crystal growing, crucibles for high-temperature use, etc. Glassy carbon is available at a cost of about $35/10g. Fullerene powder is available at a cost of about $55/10mg (99%C10). Diamond powder (99.9%) costs about $40/g. |
|
Uses |
Crucibles, retorts, foundry facings, molds, lubricants, paints and coatings, boiler compounds, powder glazing, electrotyping, monochromator in X-ray diffraction analysis, fluorinated graphite polymers with fluorine-to-carbon ratios of 0.1–1.25, electrodes, bricks, chemical equipment, motor and generator brushes, seal rings, rocket nozzles, moderator in nuclear reactors, cathodes in electrolytic cells, pencils, fibers, self-lubricating bearings, intercalation compounds. |
|
Definition |
The crystalline allotropic form of carbon. |
|
General Description |
Black grains that have been treated to improve absorptive ability. May heat spontaneously if not properly cooled after manufacture. |
|
Air & Water Reactions |
Highly flammable. Dust is explosive when exposed to heat or flame. Freshly prepared material can heat and spontaneously ignite in air. The presence of water assists ignition, as do contaminants such as oils. Insoluble in water. |
|
Reactivity Profile |
Carbon is incompatible with very strong oxidizing agents such as fluorine, ammonium perchlorate, bromine pentafluoride, bromine trifluoride, chlorine trifluoride, dichlorine oxide, chlorine trifluoride, potassium peroxide, etc. . Also incompatible with air, metals, unsaturated oils. [Lewis]. |
|
Hazard |
(Powder, natural) Fire risk. |
|
Health Hazard |
Fire may produce irritating and/or toxic gases. Contact may cause burns to skin and eyes. Contact with molten substance may cause severe burns to skin and eyes. Runoff from fire control may cause pollution. |
|
Fire Hazard |
Flammable/combustible material. May be ignited by friction, heat, sparks or flames. Some may burn rapidly with flare burning effect. Powders, dusts, shavings, borings, turnings or cuttings may explode or burn with explosive violence. Substance may be transported in a molten form at a temperature that may be above its flash point. May re-ignite after fire is extinguished. |
|
Agricultural Uses |
Carbon (C) is found in every living being as it forms the major constituent of living cells. As an essential element for plants and animals, carbon is derived from atmospheric carbon dioxide assimilated by plants and photoautotrophic microbes during photosynthesis. Carbon occurs in nature both in an elemental form and as compounds. For example, coal contains elemental carbon which, upon heating in the absence of air, loses the volatile substances, and gives coke. Both coal and coke are amorphous (non-crystalline) forms of carbon. The two crystalline forms of carbon are diamond and graphite. These are called the two allotropes of carbon. Allotropes are two or more forms of an element that exist in different physical forms, and differ in the bonding or molecular structure of their fundamental units. Carbon is found in a combined state in all living organisms, as well as in fossil fuels such as methane and petroleum. It also occurs in large amounts in carbonates such as limestone. Carbon, a non-metallic element, is found at the head of Group 14 (formerly IV) in the Periodic Table. It is unique in the variety and complexity of compounds it forms, which is due to the ability of carbon atoms to bond to one another in long chains, rings and combinations of rings and chains. Carbon in combination with H, O, N, S and other elements produces such a variety of compounds, that a separate branch of chemistry called organic chemistry, came into being around carbon compounds. Elemental carbon is a fairly inert substance. It is insoluble in water, dilute acids and bases, and organic solvents. Each carbon atom has four valence electrons and these tend to share with other atoms in the formation of four covalent bonds. Carbon forms two oxides - carbon monoxide (CO) and carbon dioxide (CO2)-which are formed when carbon or carbon-containing compounds are burned in insufficient or inexcess air, respectively. The free element has many uses, ranging from ornamental applications as diamond in jewelry to the black-colored pigment of carbon black in automobile tires and printing inks. Graphite, another form of carbon, is used for high temperature crucibles, arc lights, dry-cell electrodes, lead pencils and as a lubricant. Charcoal, an amorphous form of carbon, is used as an absorbent for gases and as a decolorizing agent in its activated form. |
|
Safety Profile |
Moderately toxic by intravenous route. Experimental reproductive effects. It can cause a dust irritation, particularly to the eyes and mucous membranes. See also CARBON BLACK, SOOT. Combustible when exposed to heat. Dust is explosive when exposed to heat or flame or oxides, peroxides, oxosalts, halogens, interhalogens, 02, (NH4NO3 + heat), (NH4ClO4 @ 240°), bromates, Ca(OCl)2, chlorates, (Cla + Cr(OCl)2), Cl0, iodates, 105, Pb(NO3)~, HgNO3, HNO3, (oils + air), (K + air), NaaS, Zn(NO3)a. Incompatible with air, metals, oxidants, unsaturated oils. |
|
Potential Exposure |
Natural graphite is used in foundry facings, steel making lubricants, refractories, crucibles, pencil “lead,” paints, pigments, and stove polish. Artificial graphite may be substituted for these uses with the excep tion of clay crucibles; other types of crucibles may be pro duced from artificial graphite. Additionally, it may be used as a high temperature lubricant or for electrodes. It is uti lized in the electrical industry in electrodes, brushes, con tacts, and electronic tube rectifier elements; as a constituent in lubricating oils and greases; to treat friction elements, such as brake linings; to prevent molds from sticking together; and in moderators in nuclear reactors. In addition, concerns have been expressed about synthetic graphite in fibrous form. Those exposed are involved in production of graphite fibers from pitch or acrylonitrile fibers and the manufacture and use of composites of plastics, metals, or ceramics reinforced with graphite fibers. |
|
Shipping |
UN1362 Carbon, activated, Hazard Class: 4.2; Labels: 4.2-Spontaneously combustible material, International. |
|
Purification Methods |
Charcoal (50g) is added to 1L of 6M HCl and boiled for 45minutes. The supernatant is discarded, and the charcoal is boiled with two more lots of HCl, then with distilled water until the supernatant no longer gives a test for chloride ion. The charcoal (now phosphate-free) is filtered onto a sintered-glass funnel and air dried at 120o for 24hours. [Lippin et al. J Am Chem Soc 76 2871 1954.] The purification can be carried out using a Soxhlet extractor (without cartridge), allowing longer extraction times. Treatment with conc H2SO4 instead of HCl has been used to remove reducing substances. |
|
Incompatibilities |
Graphite is a strong reducing agent and reacts violently with oxidizers, such as fluorine, chlorine trifluoride, and potassium peroxide. Forms an explosive mixture with air. May be spontaneously combustible in air. |
|
Waste Disposal |
Do not incinerate. Carbon (graphite) fibers are difficult to dispose of by incineration. Waste fibers should be packaged and disposed of in a land fill authorized for the disposal of special wastes of this nature, or as otherwise may be required by law. |
|
Who Evaluation |
Evaluation year: 1998 |
|
EXPOSURE ROUTES |
inhalation, skin absorption, ingestion, skin and/or eye contact |
|
FIRST AID |
(See procedures) Eye:Irrigate immediately Skin:Soap wash Breathing:Respiratory support Swallow:Medical attention immediately |
|
Consumer Uses |
ECHA has no public registered data indicating whether or in which chemical products the substance might be used. ECHA has no public registered data on the routes by which this substance is most likely to be released to the environment. |
InChI:InChI=1/C
7440-44-0 Relevant articles
Aluminium powder as a reactive template for preparation of carbon flakes from CCl4
?imon, Erik,Billik, Peter,Orov?ík, ?ubomír,Nagy, ?tefan,Sasinková, Vlasta,Palou, Martin T.,?krátek, Martin,Trembo?ová, Veronika,Plesch, Gustav
, p. 4599 - 4607 (2020)
Abstract: This work presents a simple pr...
Preparation of a platelike carbon nanomaterial using MgO as a template
Davydov,Kryukov,Gerya,Izvol'skii,Rakov
, p. 244 - 248 (2012)
A carbon nanomaterial in the form of hol...
Chemical vapor deposition of methane for single-walled carbon nanotubes
Kong, Jing,Cassell, Alan M.,Dai, Hongjie
, p. 567 - 574 (1998)
We report the synthesis of high-quality ...
A novel emulsion-based replica method for the synthesis of mesoporous carbon
Kang, Jin Kyeong,Xiong, Wei,Kang, Ji Hoon,Kang, Jukyoung,Kim, Seok,Jung, Yongju
, p. 7259 - 7262 (2018)
We present a novel approach for the synt...
STRUCTURE OF THE RESIDUAL CARBON MADE BY FLUOROCARBON PYROLYSIS
Fialkov, A. S.,Dobryakov, S. N.,Khorkhorin, A. V.,Tyan, L. S.,Polyakova, N. V.,et al.
, p. 413 - 416 (1990)
Fluorocarbon defluorination products sho...
Electrical and thermoelectric power measurements of GaInSe2 single crystals
Mobarak
, p. 1259 - 1263 (2009)
A single crystal of GaInSe2 was prepared...
Formation of carbon nanoparticles by the condensation of supersaturated atomic vapor obtained by the laser photolysis of C3O2
Gurentsov,Eremin,Schulz
, p. 194 - 203 (2007)
A new technique is suggested for obtaini...
Decomposition of methane over iron catalysts at the range of moderate temperatures: The influence of structure of the catalytic systems and the reaction conditions on the yield of carbon and morphology of carbon filaments
Ermakova,Ermakov,Chuvilin,Kuvshinov
, p. 183 - 197 (2001)
Decomposition of high-purity methane in ...
Synthesis of SiC nanorods using floating catalyst
Zhang, Yingjiu,Wang, NanLin,He, Rongrui,Chen, Xihua,Zhu, Jing
, p. 595 - 598 (2001)
The beta-silicon carbide (β-SiC) nanorod...
The surface decoration and electrochemical hydrogen storage of carbon nanofibers
Yan, Xiaoqi,Gao, Xueping,Li, Ying,Liu, Zhanquan,Wu, Feng,Shen, Yutian,Song, Deying
, p. 336 - 341 (2003)
The tube-like carbon nanofibers (CNF) wi...
Growth of graphene layers on HOPG via exposure to methyl radicals
Wellmann, Ralf,B?ttcher, Artur,Kappes, Manfred,Kohl, Ulrich,Niehus, Horst
, p. 81 - 93 (2003)
The interaction of methyl radicals with ...
Magnetotransport in the amorphous carbon films prepared from succinic anhydride
Prasad,Subramanyam
, p. 168 - 176 (2005)
In this paper, we report the low-tempera...
One-step replication and enhanced catalytic activity for cathodic oxygen reduction of the mesostructured Co3O4/carbon composites
Wang, Yongxia,Cui, Xiangzhi,Chen, Lisong,Wei, Chenyang,Cui, Fangming,Yao, Heliang,Shi, Jianlin,Li, Yongsheng
, p. 4163 - 4168 (2014)
Mesostructured Co3O4/C composites of hig...
Nanodomain structure of carbon-rich silicon carbonitride polymer-derived ceramics
Mera, Gabriela,Tamayo, Aitana,Nguyen, Hong,Sen, Sabyasachi,Riedel, Ralf
, p. 1169 - 1175 (2010)
The presence of nanodomains in polymer-d...
Synthesis of diamondlike films by an electrochemical method at atmospheric pressure and low temperature
Novikov,Dymont
, p. 200 - 202 (1997)
A technique of carbon film synthesis bas...
Synthesis and characterization of SiC nanowires through a reduction-carburization route
Hu,Lu,Tang,Deng,Jiang,Qian,Yu,Zhou,Liu,Wu
, p. 5251 - 5254 (2000)
Cubic silicon carbide (3C-SiC) nanowires...
Electronic properties of grains and grain boundaries in graphene grown by chemical vapor deposition
Jauregui, Luis A.,Cao, Helin,Wu, Wei,Yu, Qingkai,Chen, Yong P.
, p. 1100 - 1104 (2011)
We synthesize hexagonal shaped single-cr...
Synthesis of 15R polytype of diamond in oxy-acetylene flame grown diamond thin films
Kapil,Mehta,Vankar
, p. 2520 - 2522 (1996)
15R polytype of diamond has been synthes...
The development of carbon nanotubes/RuO2·χH2O electrodes for electrochemical capacitors
Ma,Wei,Xu,Liang,Wu
, p. 1813 - 1816 (2000)
Carbon nanotubes were refluxed with nitr...
One-step and template-free preparation of hierarchical porous carbons with high capacitive performance
Zhou, Jin,Zhang, Zhongshen,Li, Zhaohui,Zhu, Tingting,Zhuo, Shuping
, p. 46947 - 46954 (2015)
Considering the wide application of hier...
Catalytic growth of single-wall carbon nanotubes from metal particles
Hafner, Jason H.,Bronikowski, Michael J.,Azamian, Bobak R.,Nikolaev, Pavel,Rinzler, Andrew G.,Colbert, Daniel T.,Smith, Ken A.,Smalley, Richard E.
, p. 195 - 202 (1998)
Single-walled carbon nanotubes (SWNTs) h...
Few-layer epitaxial graphene grown on vicinal 6H-SiC studied by deep ultraviolet Raman spectroscopy
Kisoda, Kenji,Kamoi, Susumu,Hasuike, Noriyuki,Harima, Hiroshi,Morita, Kouhei,Tanaka, Satoru,Hashimoto, Akihiro
, (2010)
Few layer epitaxial graphenes (1.8-3.0 l...
Electrodeposited Ni(OH)2 nanoflakes on graphite nanosheets prepared by plasma-enhanced chemical vapor deposition for supercapacitor electrode
Wang, Xin,Wang, Yayu,Zhao, Cuimei,Zhao, Yunxiao,Yan, Baoyu,Zheng, Weitao
, p. 1902 - 1906 (2012)
Graphite nanosheets have been grown on N...
Thermal conductivity of hard carbon prepared from C60 fullerene
Smontara,Biljakovic,Staresinic,Pajic,Kozlov,Hirabayashi,Tokumoto,Ihara
, p. 160 - 162 (1996)
We report measurements of thermal conduc...
Controlled particle generation in an inductively coupled plasma
Schulze,Von Keudell,Awakowicz
, (2006)
By injecting pulses of acetylene into an...
Carbon-hydrogen bonding in near-frictionless carbon
Johnson,Woodford,Rajput,Kolesnikov,Schleuter,Eryilmaz,Erdemir
, (2008)
The uniquely low friction behavior of ne...
Melt Casting LiFeP O4: II. Particle Size Reduction and Electrochemical Evaluation
MacNeil,Devigne,Michot,Rodrigues,Liang,Gauthier
, p. A463-A468 (2010)
LiFeP O4 was prepared using a melt casti...
CO2turned into a nitrogen doped carbon catalyst for fuel cells and metal-air battery applications
?mits, Kri?jānis,?utka, Andris,Kruusenberg, Ivar,Lo?s, Jānis,Mikli, Valdek,Ratso, Sander,Vītola, Virgīnija,Walke, Peter Robert
, p. 4435 - 4445 (2021)
Heteroatom doped metal-free catalysts ar...
Monolayer graphene film/silicon nanowire array Schottky junction solar cells
Xie, Chao,Lv, Peng,Nie, Biao,Jie, Jiansheng,Zhang, Xiwei,Wang, Zhi,Jiang, Peng,Hu, Zhizhong,Luo, Linbao,Zhu, Zhifeng,Wang, Li,Wu, Chunyan
, (2011)
Schottky junction solar cells were const...
Lanthanide complexes of 3-methoxy-salicylaldehyde: Thermal and kinetic investigation by simultaneous TG/DTG-DTA coupled with MS
Papadopoulos, Christos,Kantiranis, Nikolaos,Vecchio, Stefano,Lalia-Kantouri, Maria
, p. 931 - 938 (2010)
The reaction of a lanthanide(III) nitrat...
Self-assembly of carbon nanohelices: Characteristics and field electron emission properties
Zhang, Guangyu,Jiang, Xin,Wang, Enge
, p. 2646 - 2648 (2004)
The fabrication of self-assembled carbon...
New heterometallic pivalates with FeIII and ZnII ions: Synthesis, structures, magnetic, thermal properties
Lutsenko, Irina A.,Kiskin, Mikhail A.,Efimov, Nikolay N.,Ugolkova, Elena A.,Maksimov, Yurii V.,Imshennik, Vladimir K.,Goloveshkin, Alexander S.,Khoroshilov, Andrey V.,Lytvynenko, Anton S.,Sidorov, Aleksey A.,Eremenko, Igor L.
, p. 165 - 175 (2017)
Techniques for synthesizing Zn(II)-Fe(II...
Diamondlike carbon deposition on silicon using radio-frequency inductive plasma of Ar and C2H2 gas mixture in plasma immersion ion deposition
Lee,He,Walter,Nastasi,Tesmer,Tuszewski,Tallant
, p. 2423 - 2425 (1998)
Diamondlike carbon (DLC) was deposited o...
Monolayer graphene growth on Ni(111) by low temperature chemical vapor deposition
Addou, Rafik,Dahal, Arjun,Sutter, Peter,Batzill, Matthias
, (2012)
In contrast to the commonly employed hig...
Growth of carbon nanowires and nanotubes via ultrarapid heating of ethanol vapor
Red'kin,Malyarevich
, p. 353 - 356 (2003)
A method is proposed for producing carbo...
Fluorination of single-wall carbon nanotubes
Mickelson,Huffman,Rinzler,Smalley,Hauge,Margrave
, p. 188 - 194 (1998)
Purified single-wall carbon nanotubes (S...
Synthesis of Fe (Co or Ni) loaded mesoporous carbon composites and their adsorption behaviors for methyl orange
Jiang, Tingshun,Fang, Weibing,Zhao, Qian,Liu, Wangping,Zhao, Haibo,Le, Shukun
, p. 5261 - 5270 (2017)
Mesoporous carbon (CMK-3) was synthesize...
In situ-grown carbon nanotube array with excellent field emission characteristics
Rao,Jacques,Haddon,Zhu,Bower,Jin
, p. 3813 - 3815 (2000)
In situ catalytic thermal decomposition ...
Synthesis and characterization of highly ordered Co-MCM-41 for production of aligned single walled carbon nanotubes (SWNT)
Lim, Sangyun,Ciuparu, Dragos,Pak, Chanho,Dobek, Frank,Chen, Yuan,Harding, David,Pfefferle, Lisa,Haller, Gary
, p. 11048 - 11056 (2003)
Highly ordered cobalt substituted MCM-41...
The carbon phases formed during pyrolysis of gaseous hydrocarbons in reaction volume of a fluidized bed apparatus
Kurbakov
, p. 23 - 34 (2009)
In view of the kinetic laws of hydrocarb...
DIRECT CURRENT ARC-PLASMA SYNTHESIS OF TUNGSTEN CARBIDES.
Ronsheim,Toth,Mazza,Pfender,Mitrofanov
, p. 2665 - 2674 (1981)
Chemical reactions which occur in a ther...
Structural Evolution from Metal-Organic Framework to Hybrids of Nitrogen-Doped Porous Carbon and Carbon Nanotubes for Enhanced Oxygen Reduction Activity
Zhang, Linjie,Wang, Xiuyun,Wang, Ruihu,Hong, Maochun
, p. 7610 - 7618 (2015)
A series of hybrids of nitrogen-doped gr...
Single-walled nanotubes by the pyrolysis of acetylene-organometallic mixtures
Satishkumar,Govindaraj,Sen, Rahul,Rao
, p. 47 - 52 (1998)
Gas-phase pyrolysis of acetylene along w...
Preparation of hollow carbon nanospheres at low temperature via new reaction route
Ni, Youbao,Shao, Mingwang,Tong, Yanhua,Qian, Guixiang,Wei, Xianwen
, p. 908 - 911 (2005)
Hollow carbon nanospheres were obtained ...
High yield of single-wall carbon nanotubes by arc discharge using Rh-Pt mixed catalysts
Saito, Yahachi,Tani, Yoshihiko,Miyagawa, Norihisa,Mitsushima, Koichi,Kasuya, Atsuo,Nishina, Yuichiro
, p. 593 - 598 (1998)
Single-walled carbon nanotubes (SWNT) we...
Methane Decomposition on the Surface of Molybdenum Nanoparticles at Room Temperature
Eremin, A. V.,Grigoriev, Yu. V.,Gurentsov, E. V.,Khmelenin, D. N.,Kolotushkin, R. N.
, p. 224 - 231 (2020)
Abstract: The decomposition of methane o...
Synthesis, evaluation, and kinetic assessment of Co-based catalyst for enhanced methane decomposition reaction for hydrogen production
Al Mesfer, Mohammed K.,Danish, Mohd,Shah, Mumtaj
, p. 90 - 103 (2021/10/19)
In this work, the development of 10, 30,...
Liquid-Metal-Enabled Mechanical-Energy-Induced CO2 Conversion
Tang, Junma,Tang, Jianbo,Mayyas, Mohannad,Ghasemian, Mohammad B.,Sun, Jing,Rahim, Md Arifur,Yang, Jiong,Han, Jialuo,Lawes, Douglas J.,Jalili, Rouhollah,Daeneke, Torben,Saborio, Maricruz G.,Cao, Zhenbang,Echeverria, Claudia A.,Allioux, Francois-Marie,Zavabeti, Ali,Hamilton, Jessica,Mitchell, Valerie,O'Mullane, Anthony P.,Kaner, Richard B.,Esrafilzadeh, Dorna,Dickey, Michael D.,Kalantar-Zadeh, Kourosh
, (2021/10/25)
A green carbon capture and conversion te...
Antibacterial and anticorrosion behavior of bioactive complexes of selected transition metal ions with new 2-acetylpyridine Schiff base
Ashmawy, Ashraf M.,Deghadi, Reem G.,Elsharkawy, Ahmed E.,Mohamed, Gehad G.
, (2022/01/19)
Successful preparation of Schiff base 4-...
Self-redox reaction of carbon in molten salt for anode materials of lithium/sodium-ion batteries
Chen, Denghui,Ning, Zhiqiang,Song, Qiushi,Xie, Hongwei,Zhao, Hengpeng
, (2022/04/03)
Preparation of carbonaceous anode with e...
7440-44-0 Process route
-
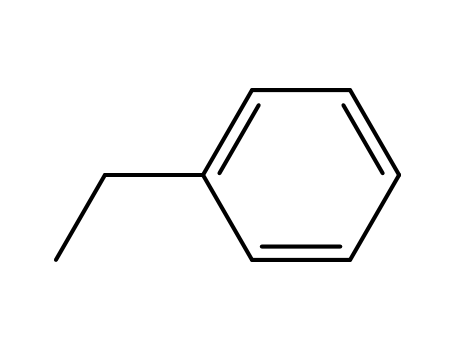
- 100-41-4,27536-89-6
ethylbenzene

-
tar
-
tar

-
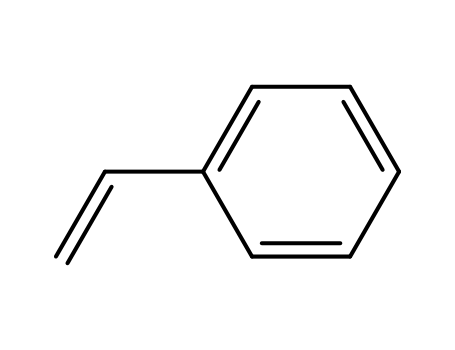
- 100-42-5,25038-60-2,25247-68-1,28213-80-1,28325-75-9,79637-11-9,9003-53-6
styrene

-
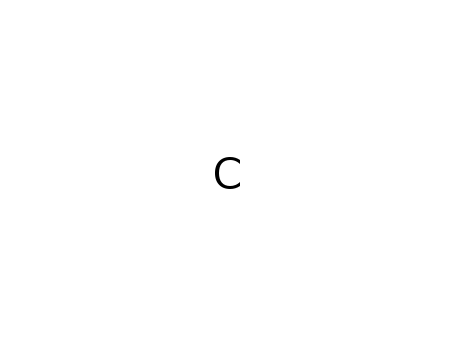
- 7440-44-0
pyrographite

-
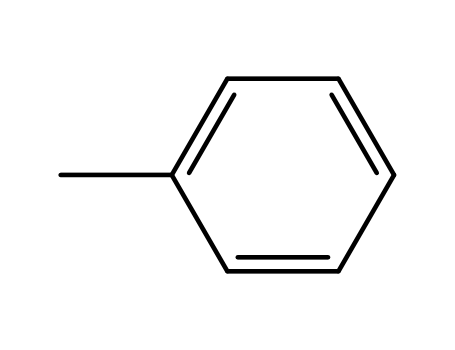
- 108-88-3,15644-74-3,16713-13-6
toluene

-
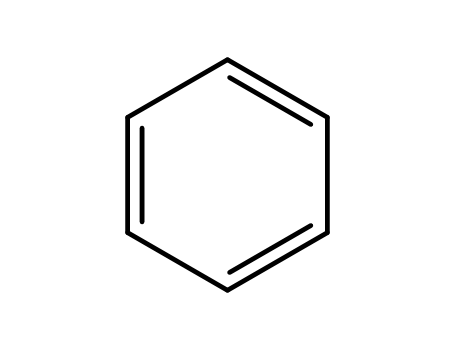
- 71-43-2,26181-88-4,54682-86-9,13967-78-7,174973-66-1
benzene

-
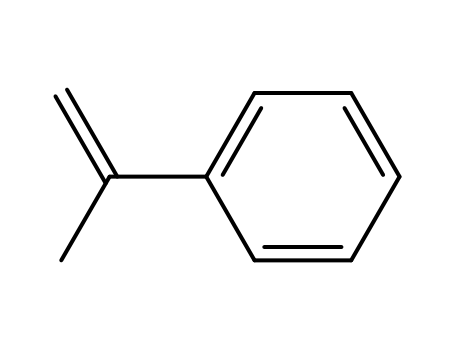
- 98-83-9,6144-04-3,25014-31-7,42612-14-6
isopropenylbenzene
| Conditions | Yield |
|---|---|
|
iron(III) nitrate (3 wtpercent as Fe2O3); potassium nitrate (3 wtpercent as K2O); yttrium nitrate (1.1 wtpercent as Y2O3); alumina; mixture of, calcined; at 548 - 598 ℃; Product distribution / selectivity; Continuous reaction;
|
-
- 34557-54-5,27936-85-2
methane

-
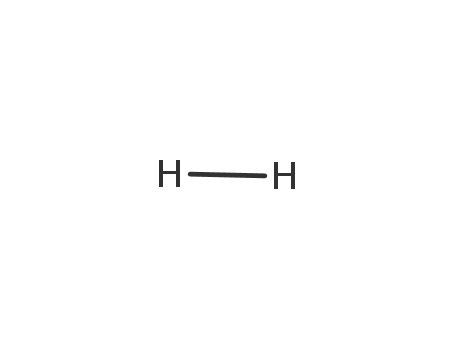
- 1333-74-0
hydrogen

-

- 7440-44-0
pyrographite
| Conditions | Yield |
|---|---|
|
With reduced Ni-Mo catalyst supported on CeO2; at 700 ℃; for 0.166667h; under 760.051 Torr; Reagent/catalyst; Time;
|
73.4% |
|
metal alloy; at 80 - 175 ℃; for 0.25 - 0.253333h; Conversion of starting material;
|
|
|
Ni65Cu25; In neat (no solvent); pure CH4 at atmospheric pressure heated with catalyst powder at 723-1023K up to 120 h until methane conversion decreased to about 3%;
|
|
|
Ni65Cu10(Nb2O5)10; In neat (no solvent); pure CH4 at atmospheric pressure heated with catalyst powder at 723-1023K up to 120 h until methane conversion decreased to about 3%;
|
|
|
Ni65Cu10(Nb2O5)5; In neat (no solvent); pure CH4 at atmospheric pressure heated with catalyst powder at 723-1023K up to 120 h until methane conversion decreased to about 3%;
|
|
|
Ni65Cu10(Nb2O5)17; In neat (no solvent); pure CH4 at atmospheric pressure heated with catalyst powder at 723-1023K up to 120 h until methane conversion decreased to about 3%;
|
|
|
Ni65Cu10(Nb2O5)35; In neat (no solvent); pure CH4 at atmospheric pressure heated with catalyst powder at 723-1023K up to 120 h until methane conversion decreased to about 3%;
|
|
|
1000°C, nearly complete decompn.;
|
|
|
860-1100°C, equil.;
|
|
|
at 1100-1278 °C on platinum band covered with earth alkali oxides on one side;
|
|
|
at 1200 °C;
|
|
|
compn. of equilibrium CH4-H2 mixts. at 300-800 °C;
|
|
|
part of CH4 burned to reach temp. of decompn.;
|
|
|
|
|
|
With air; CH4 partly burned, partly thermally decompd.; N2-H2 mixt. obtained;
|
|
|
With catalyst: Ni(x)Cu(y)Mg(z)O; In neat (no solvent); at 665°C; catalyst Ni(x)Cu(y)Mg(z)O (x:y:z = 2.4:0.8:1); XRD;
|
|
|
With Ni on Fe2O3/Al2O3; at 850 ℃; Equilibrium constant; Inert atmosphere;
|
|
|
at 635 ℃; Temperature; Pyrolysis;
|
|
|
With Ni-doped silica; at 550 ℃; for 10h; Reagent/catalyst; Time; Catalytic behavior;
|
|
|
With Co/TiO2-Al2O3; at 600 ℃; under 750.075 Torr; Temperature; Kinetics; Flow reactor;
|
7440-44-0 Upstream products
-
91-20-3
naphthalene
-
92-52-4
biphenyl
-
85-01-8
phenanthrene
-
120-12-7
anthracene
7440-44-0 Downstream products
-
55760-23-1
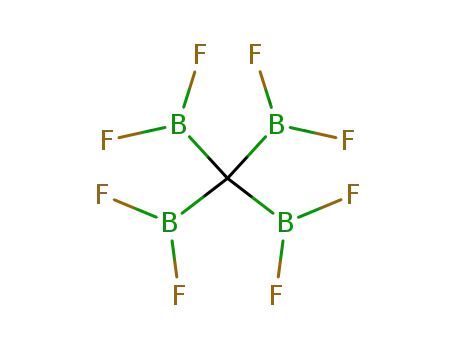
tetrakis(difluoroboryl)methane
-
23423-53-2
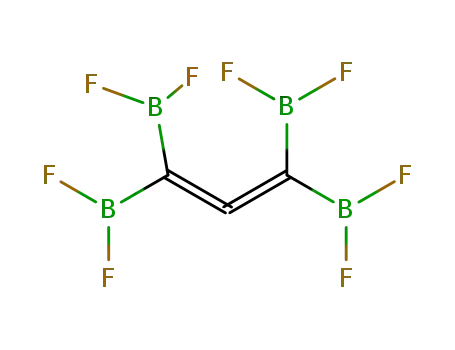
tetrakis(difluoroboryl)allene
-
50-00-0
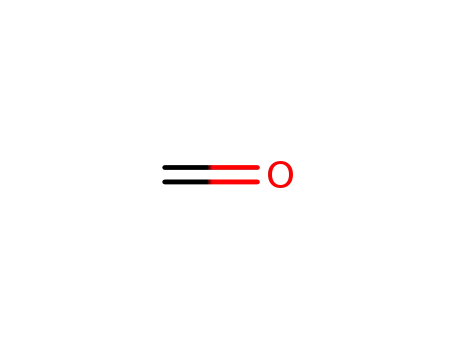
formaldehyd
-
74-86-2
acetylene
Relevant Products
-
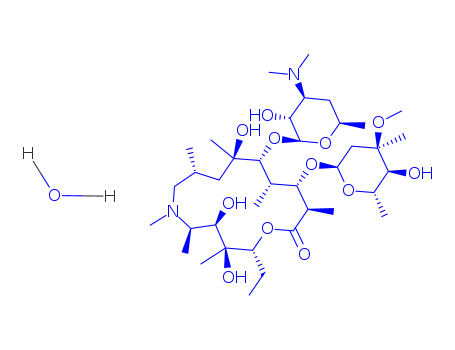
Azithromycin dihydrate
CAS:117772-70-0
-
Sodium thiocyanate
CAS:540-72-7
-
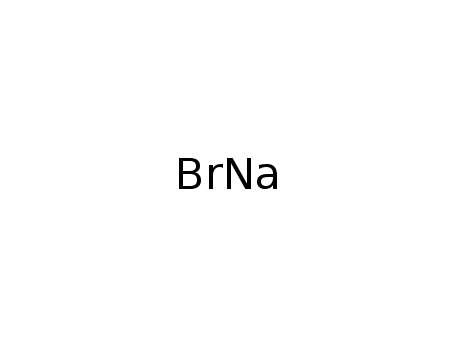
Sodium bromide
CAS:7647-15-6