7647-15-6
- Product Name:Sodium bromide
- Molecular Formula:NaBr
- Purity:99%
- Molecular Weight:102.894
Product Details;
CasNo: 7647-15-6
Molecular Formula: NaBr
Appearance: white powder
Buy Reliable Quality High Purity Sodium bromide 7647-15-6 Efficient Delivery
- Molecular Formula:NaBr
- Molecular Weight:102.894
- Appearance/Colour:white powder
- Melting Point:755 °C(lit.)
- Refractive Index:1.6412
- Boiling Point:1390 °C
- Flash Point:1390°C
- PSA:0.00000
- Density:3.203 g/cm3
- LogP:-2.99600
Sodium bromide(Cas 7647-15-6) Usage
Sodium bromide is a salt commonly employed as a source of bromide ions in chemical reactions. Its versatile applications span different industries. Historically used in the medical field as a sedative and anticonvulsant, its medical use has diminished with the introduction of safer alternatives. In the oil and gas industry, sodium bromide serves as a dense brine fluid in drilling fluids, contributing to wellbore stability and preventing blowouts. In photography, it plays a crucial role in silver bromide emulsions for traditional black-and-white photography. Additionally, sodium bromide is utilized in chemical synthesis processes and functions as a disinfectant in water treatment, particularly in hot tubs and swimming pools, where it effectively controls microbial growth, ensuring water quality. Despite evolving applications, sodium bromide remains relevant in various fields due to its unique properties and functions.
InChI:InChI=1/BrH.Na/h1H;/q;+1/p-1
7647-15-6 Relevant articles
Self‐Diffusion of Sodium in Sodium Chloride and Sodium Bromide
Dillon Mapother; H. Nelson Crooks; Robert Maurer
, J. Chem. Phys. 18, 1231–1236 (1950)
The self‐diffusion coefficient of sodium in sodium chloride and sodium bromide has been measured as a function of temperature. A comparison of the self‐diffusion coefficient with the electrolytic conductivity reveals that the Einstein relation is satisfied at high but not at low temperatures. The temperature dependence of the diffusion coefficient may be explained by the Schottky‐Wagner vacancy theory if the presence of impurity ions is taken into account. A possible explanation for the failure of the Einstein relation in terms of association between multivalent foreign ions and vacancies is suggested.
Evaluation of kinetic parameters for the thermal decomposition of γ-irradiated sodium bromate by dynamic thermogravimetry
Nair,Malayil, Koshy Kunju,Jacob, P.Daisamma
, p. 61 - 68 (1989)
The thermal decomposition of γ-irradiate...
Ion Exchange of Layered Alkali Titanates (Na2Ti3O7, K2Ti4O9, and Cs2Ti5O11) with Alkali Halides by the Solid-State Reactions at Room Temperature
Ogawa, Makoto,Saothayanun, Taya Ko,Sirinakorn, Thipwipa Tip
, p. 4024 - 4029 (2020/04/08)
Ion exchange of layered alkali titanates...
Synthesis, characterization, and catalytic behavior of methoxy- and dimethoxy-substituted pyridinium-type ionic liquids
Manikandan, Chitrarasu,Ganesan, Kilivelu
, p. 3362 - 3367 (2014/12/11)
Synthesis of methoxy-substituted pyridin...
7647-15-6 Process route
-

- 7726-95-6
bromine

-
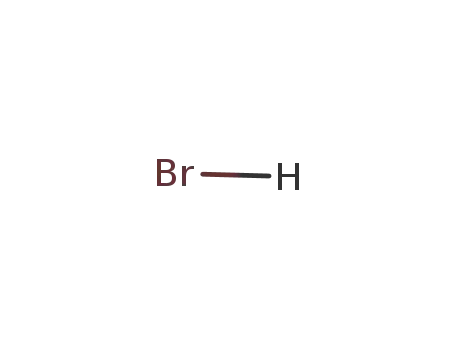
- 10035-10-6,12258-64-9
hydrogen bromide

-
- 7647-15-6
sodium bromide
| Conditions | Yield |
|---|---|
|
With sodium azide; water; In water; byproducts: H2O, N2;
|
|
|
With NaN3; H2O; In water; byproducts: H2O, N2;
|
-

- 7726-95-6
bromine

-
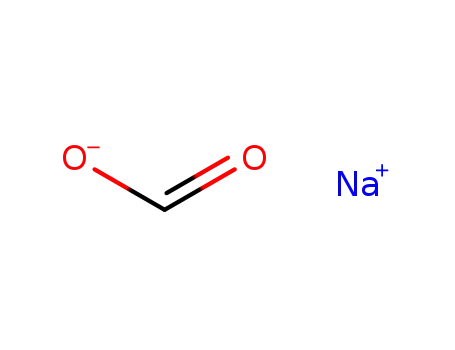
- 141-53-7
sodium formate

-
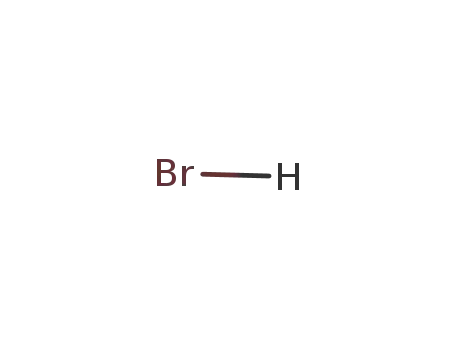
- 10035-10-6,12258-64-9
hydrogen bromide

-

- 7647-15-6
sodium bromide
| Conditions | Yield |
|---|---|
|
byproducts: CO2; fast reaction in the presence of ZnO;
|
7647-15-6 Upstream products
-
134509-56-1
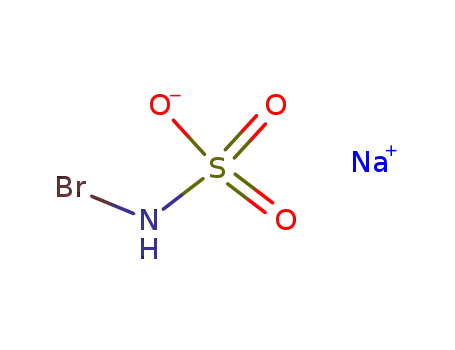
N-bromosulfamic acid sodium salt
-
10035-10-6
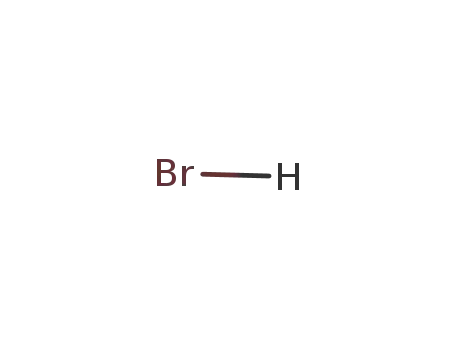
hydrogen bromide
-
773837-37-9
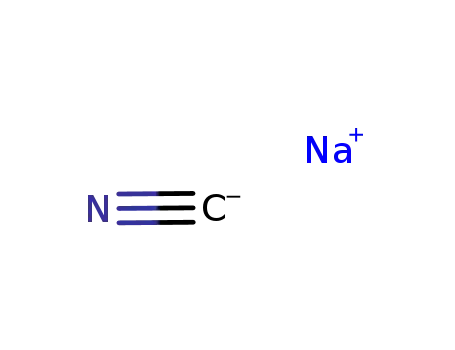
sodium cyanide
7647-15-6 Downstream products
-
10097-32-2

bromine
-
7440-23-5

sodium
-
7787-70-4

copper(I) bromide
-
7726-95-6

bromine
Relevant Products
-
Sodium tetraborate
CAS:1330-43-4
-
Carbon
CAS:7440-44-0
-
Camphor powder
CAS:21368-68-3