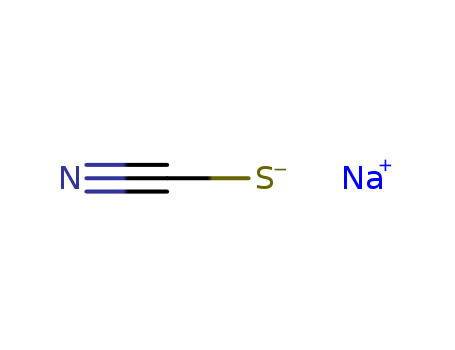
540-72-7
- Product Name:Sodium thiocyanate
- Molecular Formula:NaSCN
- Purity:99%
- Molecular Weight:81.0735
Product Details;
CasNo: 540-72-7
Molecular Formula: NaSCN
Appearance: white crystalline powder
Buy High Grade Best Quality Sodium thiocyanate 540-72-7 Fast Shipping
- Molecular Formula:NaSCN
- Molecular Weight:81.0735
- Appearance/Colour:white crystalline powder
- Vapor Pressure:2700mmHg at 25°C
- Melting Point:287 °C (dec.)(lit.)
- Boiling Point:146 °C at 760 mmHg
- Flash Point:42.1 °C
- PSA:49.09000
- Density:1.295 g/mL at 20 °C
- LogP:0.66498
Sodium thiocyanate(Cas 540-72-7) Usage
|
Chemical Properties |
Sodium thiocyanate, a white rhombic system crystal, is soluble in water, ethanol, acetone; Relative density is 1.735. Melts at approx. 287℃. Decomposes on heating and under influence of light producing toxic fumes of sulfur oxides,nitrogen oxides and cyanides. Reacts violently with acids,strong bases and strong oxidants. |
|
Uses |
Sodium thiocyanate is a valuable chemical raw material and has important uses in various sectors of the national economy. Sodium thiocyanate is mainly used as a solvent for spinning acrylic fibers, chemical analysis reagents, color film film rinses, certain plant defoliants, and airport road herbicides. Sodium thiocyanate is also used in pharmaceuticals, printing and dyeing, rubber processing, black nickel plating, and artificial mustard. Oil, etc. The main industrial production processes are: separation of sodium thiocyanate from waste liquid in coke oven gas, synthesis of sodium thiocyanate with sodium cyanide and sulfur, and production of sodium thiocyanate by metathesis reaction of ammonium thiocyanate and sodium hydroxide. The synthesis of sodium thiocyanate requires high purity HCN as raw material, which is costly and harsh. |
|
Physical properties |
Colorless crystals or white powder; deliquescent; melts at 287°C; very soluble in water; soluble in alcohol. |
|
Definition |
ChEBI: An organic sodium salt which is the monosodium salt of thiocyanic acid. |
|
Preparation |
Sodium thiocyanate is prepared by boiling an aqueous solution of sodium cyanide with sulfur: NaCN + S → NaSCN. |
|
Reactions |
Sodium thiocyanate is an analytical reagent for measuring iodide. Other uses are dyeing and printing textiles, preparing thiocyanate salts, and nickel plating. Used in titrimetry. |
|
General Description |
Odorless white solid. Sinks and mixes with water. |
|
Air & Water Reactions |
Water soluble. |
|
Reactivity Profile |
Nitric acid violently oxidized a thiocyanate solution [Bretherick 1979 p. 121]. Caution should be exercised in treating a thiocyanate with an oxidizing agent such as a peroxide or chlorate as such mixtures have been known to explode. Special Hazards of Combustion Products: Irritating oxides of sulfur and nitrogen may form in fire [USCG, 1999]. Carbonyl sulfide is produced in a violent reaction by the mixture of sulfuric acid and Sodium thiocyanate. |
|
Health Hazard |
Inhalation of dust causes irritation of nose and throat. Ingestion of large doses causes vomiting, extreme cerebral excitement, convulsions, and death in 10-48 hrs.; chronic poisoning can cause flu-like symptoms, skin rashes, weakness, fatigue, vertigo, nausea, vomiting, diarrhea, confusion. Contact with eyes causes irritation. Prolonged contact with skin may produce various skin eruptions, dizziness, cramps, nausea, and mild to severe disturbance of the nervous system. |
|
Fire Hazard |
Special Hazards of Combustion Products: Irritating oxides of sulfur and nitrogen may form in fire. |
|
Flammability and Explosibility |
Nonflammable |
|
Safety Profile |
Poison by ingestion, intravenous, and subcutaneous routes. Moderately toxic by intraperitoneal route. Large doses taken internally cause vomiting, convulsions. Chronic poisoning is manifested by weakness, confusion, diarrhea, and skin rashes. When heated to decomposition it emits very toxic fumes of NOx, SOx, and Na2O. See also THIOCYANATES. |
|
Purification Methods |
It is recrystallised from EtOH or Me2CO, and the mother liquor is removed from the crystals by centrifugation. It is very deliquescent and should be kept in an oven at 130o before use. It can be dried in a vacuum at 120o/P2O5 [Partington & Winterton Trans Faraday Soc 30 1104 1934]. Its solubility in H2O is 113% at 10o, 178% at 46o, 225.6% at 101.4o; in MeOH 35% at 15.8o, 51% at 48o, 53.5% at 52.3o; in EtOH 18.4% at 18.8o, 24.4% at 70.9o; and in Me2CO 6.85% at 18.8o and 21.4% at 56o [Hughes & Mead J Chem Soc 2282 1929]. Sodium thiocyanate has also been recrystallised from water, acetonitrile or from MeOH using Et2O for washing, then dried at 130o, or dried under vacuum at 60o for 2days. [Strasser et al. J Am Chem Soc 107 789 1985, Szezygiel et al. J Am Chem Soc 91 1252 1987.] (The latter purification removes material reacting with iodine.) Sodium thiocyanate solutions can be freed from traces of iron by repeated batch extractions with Et2O. |
|
Who Evaluation |
Evaluation year: 1985 |
InChI:InChI=1/CNS.Na/c2-1-3;/q-1;+1
540-72-7 Relevant articles
Microdetection of carbon in organic and inorganic compounds by ignition with sodium amide.
MOMOSE,UEDA,MUKAI
, p. 322 - 322 (1958)
-
COMPOUNDS CONTAINING HYDRIDO-TRICYANO-BORATE ANIONS
-
Page/Page column 52, (2013/02/28)
The present invention relates to compoun...
Kinetic study of an autocatalytic reaction: Nitrosation of formamidine disulfide
Francisco, Vitor,Garcia-Rio, Luis,Antonio Moreira, Jose,Stedman, Geoffrey
body text, p. 2292 - 2298 (2009/03/11)
The reaction kinetics for the acid nitro...
Recovery of sodium thiocynate from industrial process solution using nanofiltration technique
-
Page/Page column 6-11, (2008/06/13)
The present invention relates to a membr...
Vanadium nitride functionalization and denitrogenation by carbon disulfide and dioxide
Brask, Justin K.,Dura-Vila, Victor,Diaconescu, Paula L.,Cummins, Christopher C.
, p. 902 - 903 (2007/10/03)
A dramatic difference in behavior is obs...
540-72-7 Process route
-
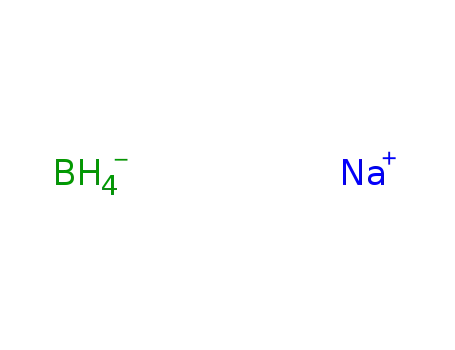
- 16940-66-2
sodium tetrahydroborate

-
-
2K(1+)*Zn(2+)*4NCS(1-)=K2{Zn(NCS)4}

-
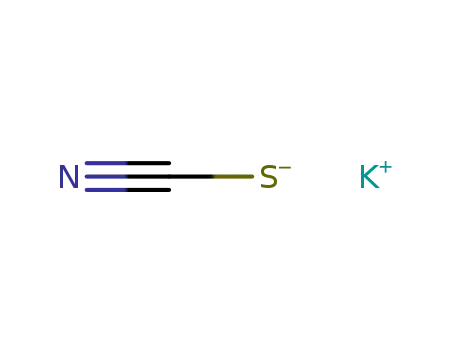
- 333-20-0
potassium thioacyanate

-
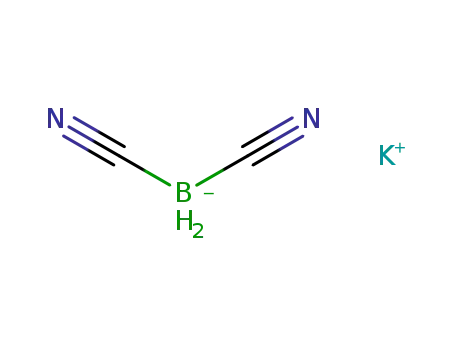
-
potassium dicyanodihydridoborate

-
![K[HB(CN)3]](/upload/2024/1/de89f96a-445d-437c-a99b-cf645e7e62e2.png)
-
K[HB(CN)3]

-
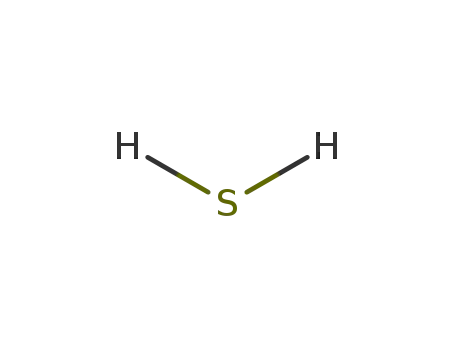
- 7783-06-4
hydrogen sulfide

-
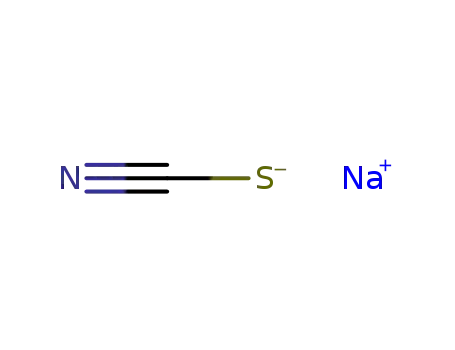
- 540-72-7
sodium thiocyanide

-

-
zinc sulfide
| Conditions | Yield |
|---|---|
|
at 185 ℃; for 5h; Under vacuum;
|
-
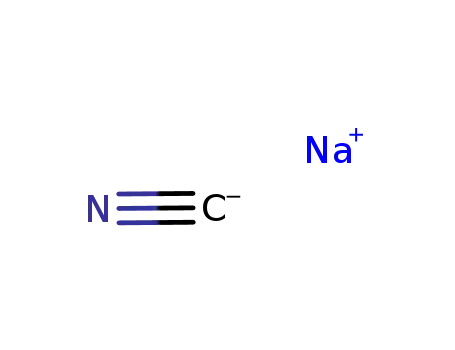
- 773837-37-9
sodium cyanide

-
-
sodium tetrathiocarbonate *3H2O

-
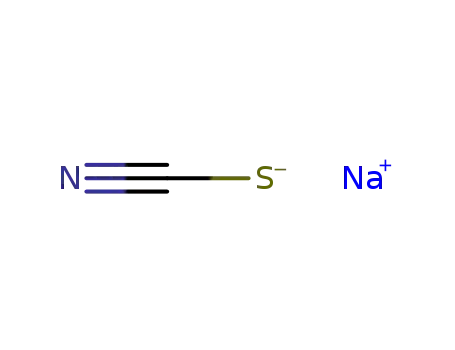
- 540-72-7
sodium thiocyanide

-
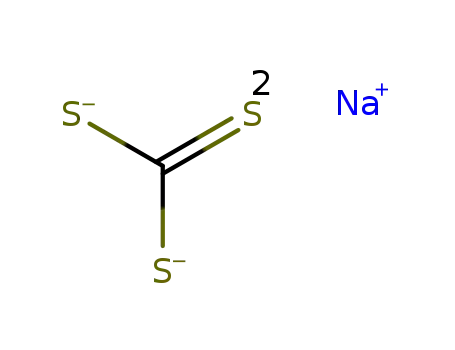
- 534-18-9
sodium trithiocarbonate
| Conditions | Yield |
|---|---|
|
In water;
|
540-72-7 Upstream products
-
75-15-0
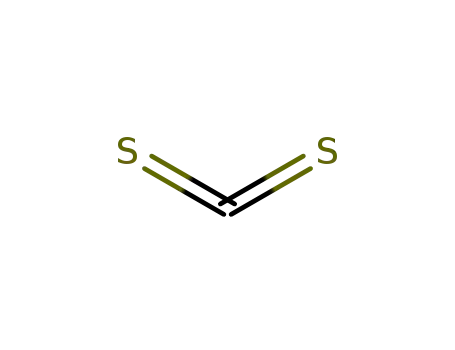
carbon disulfide
-
420-04-2
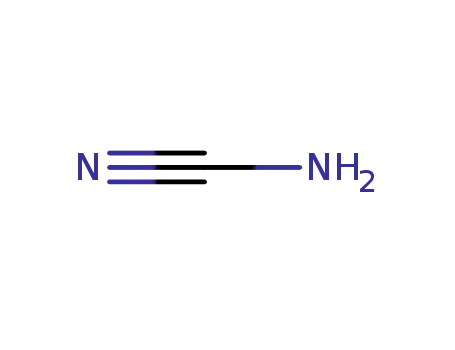
CYANAMID
-
143-33-9
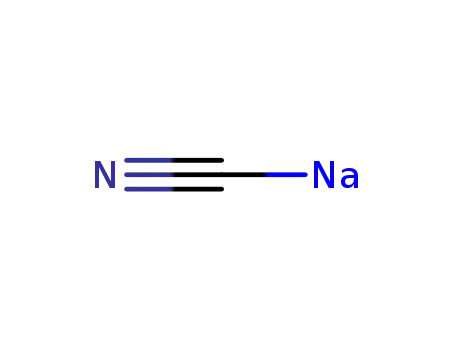
sodium cyanide
-
1147550-11-5
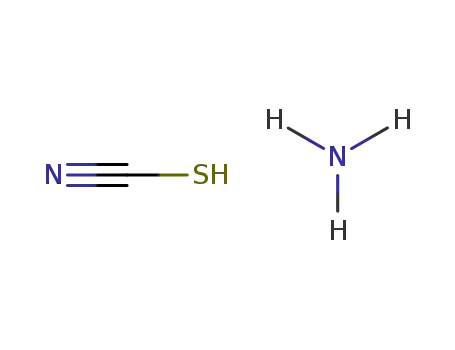
ammonium thiocyanate
540-72-7 Downstream products
-
24277-44-9
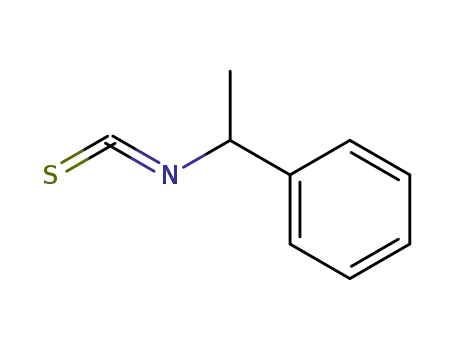
(1-isothiocyanato-ethyl)-benzene
-
79528-83-9
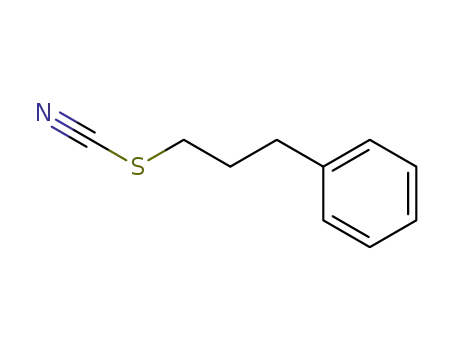
(3-thiocyanatopropyl)benzene
-
2290-65-5
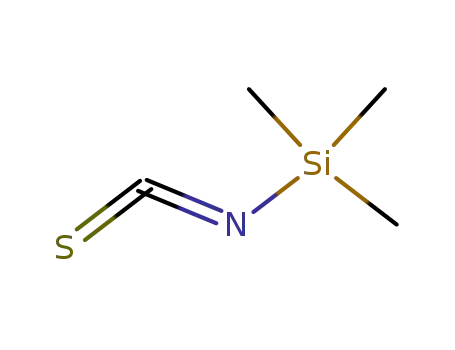
trimethylsilyl isothiocyanate
-
36234-62-5
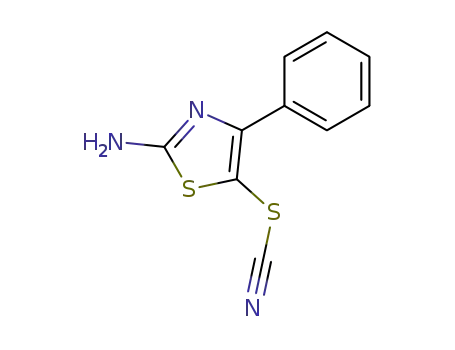
4-phenyl-5-thiocyanato-2-aminothiazole
Relevant Products
-
Sodium formaldehyde bisulfite
CAS:870-72-4
-
Ammonium chloride
CAS:12125-02-9
-
Carbon
CAS:7440-44-0