870-72-4
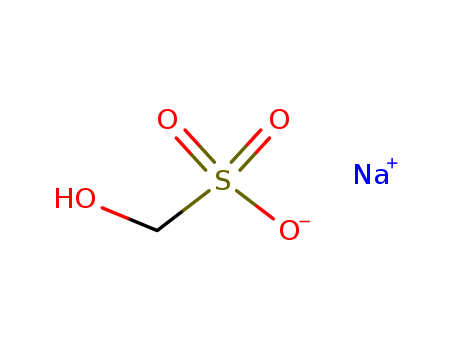
- Product Name:Sodium formaldehyde bisulfite
- Molecular Formula:CH3NaO4S
- Purity:99%
- Molecular Weight:134.088
Product Details;
CasNo: 870-72-4
Molecular Formula: CH3NaO4S
Appearance: white to almost white powder
Buy Reliable Quality Sodium formaldehyde bisulfite,Wholesale 870-72-4 Cheap Price
- Molecular Formula:CH3NaO4S
- Molecular Weight:134.088
- Appearance/Colour:white to almost white powder
- Melting Point:200 °C (dec.)(lit.)
- Boiling Point:184 °C
- Flash Point:184 °C
- PSA:85.81000
- LogP:-0.43780
Sodium formaldehyde bisulfite(Cas 870-72-4) Usage
|
Description |
Sodium Formaldehyde Bisulfite is a versatile chemical used as a raw material and intermediate in various industries, including pharmaceuticals, agrochemicals, dyestuffs, and organic synthesis. It plays a crucial role in enhancing the properties of lubricant oils through the preparation of oil-soluble sulfonate additives. Additionally, it is involved in the synthesis of specific chemicals like 3-Hydroxypropionaldehyde and is employed in complexing metal ions, acting as a pharmaceutical intermediate, and contributing to the preparation of chiral catalysts and dispersants for gypsum past. |
|
Uses |
Formaldehyde Sodium Bisulfite is used in the preparation of oil-soluble sulfonate additives used in improving anticorrosive, dispersant and antioxygenic properties of lubricant oils. Also used in chemical reactions in the preparation of chiral salen Mn(III) catalysts or lignosulfonates as dispersant for gypsum paste. |
| Chiral Salen Mn(III) Catalysts | Used in chemical reactions for the preparation of chiral salen Mn(III) catalysts. |
InChI:InChI=1/CH2O.Na.H2O3S/c1-2;;1-4(2)3/h1H2;;(H2,1,2,3)/q;+1;/p-2
870-72-4 Relevant articles
Manifesto for the routine use of NMR for the liquid product analysis of aqueous CO2 reduction: From comprehensive chemical shift data to formaldehyde quantification in water
Boutin, Etienne,Chatterjee, Tamal,Robert, Marc
supporting information, p. 4257 - 4265 (2020/04/17)
CO2 reduction research is at a critical ...
A mechanistic study of the reactions of formaldehyde with aniline in the presence of sulfite
Atherton, John H.,Brown, Kathryn H.,Crampton, Michael R.
, p. 941 - 946 (2007/10/03)
1H NMR results are reported for the reac...
TRIMETHYLAMMONIOMETHANESULFINATE AND TRIMETHYLAMMONIOMETHANESULFONATE, THE SIMPLEST SULFINIC AND SULFONIC ACID BETAINES. REVISION OF THE STRUCTURE OF THE TRIMETHYLAMINE OXIDE-SULFUR DIOXIDE PRODUCT
King, J. F.,Skonieczny, S.
, p. 11 - 20 (2007/10/02)
Bromomethanesulfinic acid (1) reacted wi...
870-72-4 Process route
-
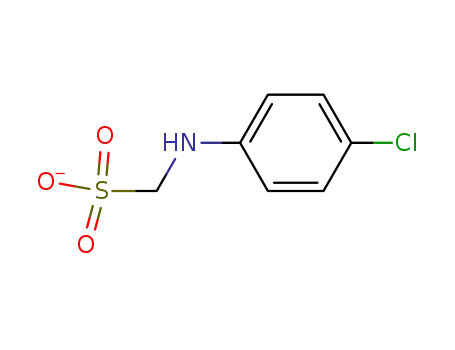
- 56638-59-6
p-chloroanilinomethanesulfonate

-
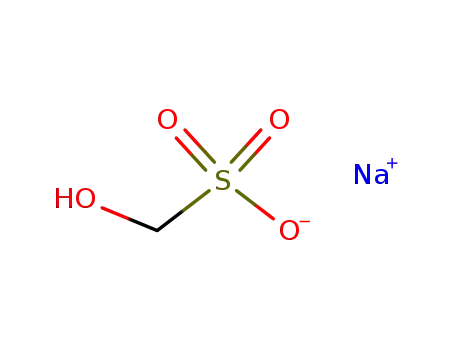
- 870-72-4
sodium formaldehyde bisulfite

-
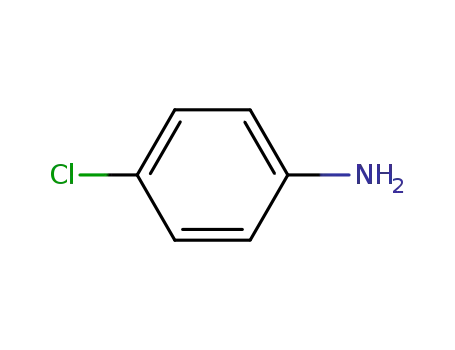
- 106-47-8
4-chloro-aniline
| Conditions | Yield |
|---|---|
|
With water; Equilibrium constant; Rate constant; Thermodynamic data; Ambient temperature; also formation constants;
|
-
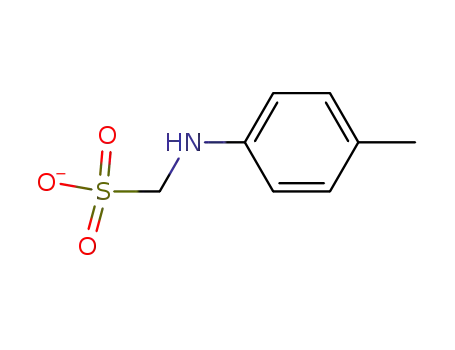
- 56638-52-9
p-methylanilinomethanesulfonate

-

- 870-72-4
sodium formaldehyde bisulfite

-
- 106-49-0,12221-03-3
p-toluidine
| Conditions | Yield |
|---|---|
|
With water; Equilibrium constant; Rate constant; Thermodynamic data; Ambient temperature; also formation constants;
|
870-72-4 Upstream products
-
50-00-0
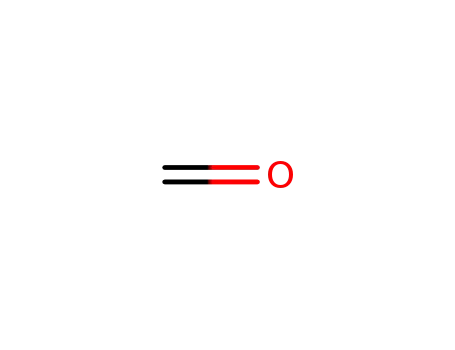
formaldehyd
-
56638-52-9

p-methylanilinomethanesulfonate
-
56638-59-6

p-chloroanilinomethanesulfonate
-
91598-30-0
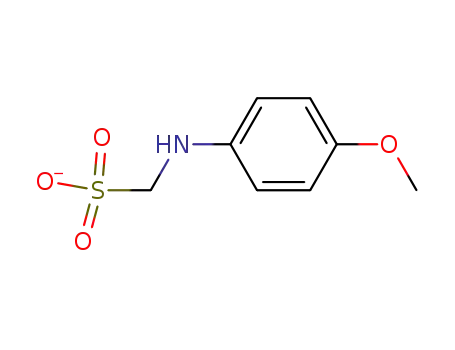
p-methoxyanilinomethanesulfonate
870-72-4 Downstream products
-
117890-30-9
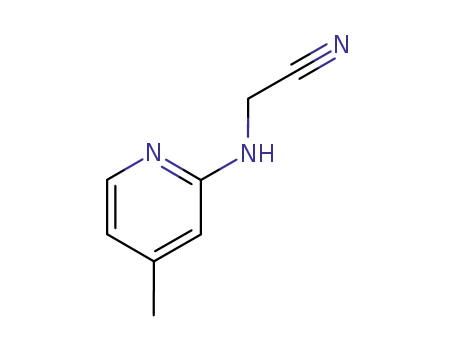
N-(4-methyl-[2]pyridyl)-glycine-nitrile
-
75241-35-9
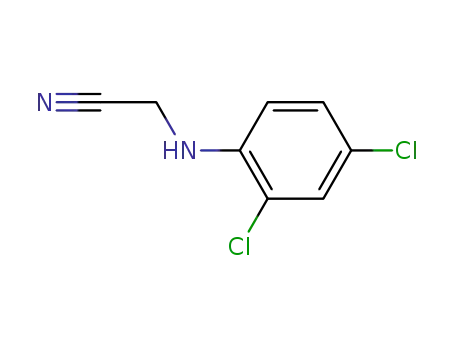
N-(2,4-dichloro-phenyl)-glycine nitrile
-
116568-41-3
(2,4-dichloro-phenylamino)-methanesulfonic acid ; sodium-salt
-
16728-84-0

p-tolylaminoacetonitrile
Relevant Products
-
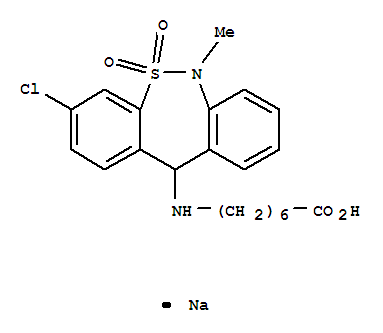
Tianeptine sodium salt
CAS:30123-17-2
-
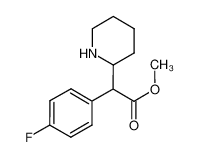
4F-MPH
CAS:1354631-33-6
-
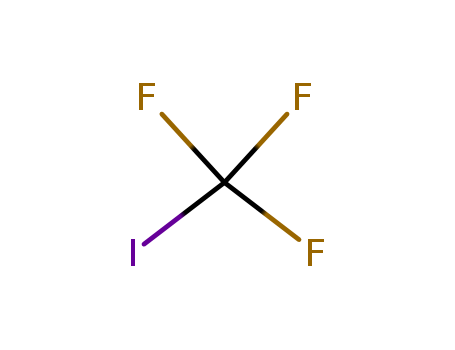
Trifluoromethyl iodide
CAS:2314-97-8