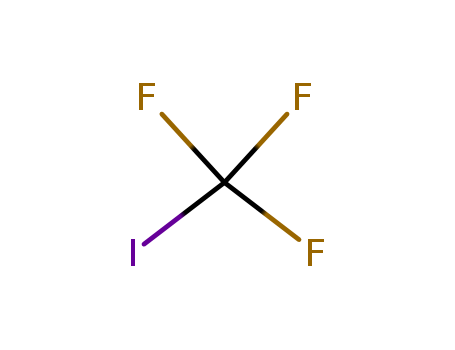
2314-97-8
- Product Name:Trifluoromethyl iodide
- Molecular Formula:CF3I
- Purity:99%
- Molecular Weight:195.911
Product Details;
CasNo: 2314-97-8
Molecular Formula: CF3I
Appearance: colourless gas
Buy High Quality Trifluoromethyl iodide 2314-97-8 On Stock
- Molecular Formula:CF3I
- Molecular Weight:195.911
- Appearance/Colour:colourless gas
- Vapor Pressure:0.0115mmHg at 25°C
- Melting Point:<?78°C(lit.)
- Refractive Index:1.379
- Boiling Point:-21.111 °C at 760 mmHg
- Flash Point:-26.936 °C
- PSA:0.00000
- Density:2.44 g/cm3
- LogP:1.94120
Trifluoromethyl iodide(Cas 2314-97-8) Usage
| Description | Trifluoromethyl Iodide, also known as Trifluoroiodomethane, is explored as an experimental alternative to Halon 1301 for gaseous fire suppression in unoccupied areas. It has specific applications in addressing in-flight aircraft and electronic equipment fires. Additionally, it has been utilized in synthetic chemistry for nucleophilic trifluoromethylation, though the requirement of ultrasound has limited its adoption in this context. The chemical is positioned as an environmentally conscious option compared to Halon 1301. |
|
Uses |
Trifluoroiodomethane is used as a gaseous fire suppression flooding agent for in-flight aircraft and electronic equipment fires. It is also an important raw material and intermediate used in organic synthesis, pharmaceuticals and agrochemicals. It is involved in the rhodium-catalyzed alfa-trifluoromethylation of alfa,beta-unsaturatedketones. It plays an important role as catalyst in the enantioselective alfa -trifluoromethylation of aldehydes through photoredox organocatalysis using a readily available iridium photocatalyst. |
|
Reactions |
Trifluoromethyl iodide reacts with [AuMeL] to give [AuMe2(CF3)L] and [AuIL](L = PMe3 or PMe2Ph), or [Au(CF3)L] and Mel (L = PPh3), or a mixture of these products (L = PMePh2). In some cases reaction of [AuMe(PMe3)] with CF3I gives [AuMe(CF3)I(PMe3)]. Evidence is presented that the reactions proceed, at least in part, by a free-radical chain mechanism. |
|
Chemical Properties |
colourless gas |
|
Synthesis Reference(s) |
Journal of the American Chemical Society, 107, p. 5014, 1985 DOI: 10.1021/ja00303a042 |
|
Flammability and Explosibility |
Notclassified |
InChI:InChI=1/C8H3ClF3NS/c9-7-2-1-5(13-4-14)3-6(7)8(10,11)12/h1-3H
2314-97-8 Relevant articles
Chemical Radical Synthesis in Gas Mixtures Induced by Infrared Multiple-Photon Dissociation
Bagratashvili, V. N.,Kuzmin, M. V.,Letokhov, V. S.
, p. 5780 - 5786 (1984)
Some experimental approaches to gas-phas...
Preparation and properties of ZnBr(CF3)*2L - a convenient route for the preparation of CF3I
Naumann, Dieter,Tyrra, Wieland,Kock, Birgit,Rudolph, Werner,Wilkes, Bernd
, p. 91 - 94 (1994)
ZnBr(CF3)*2L (L=DMF, CH3CN) can easily b...
Preparation of trifluoroiodomethane via vapour-phase catalytic reaction between pentafluoroethane and iodine
Mao, Aiqin,Wang, Hua,Tan, Linhua,Nin, Xiangyang,Pan, Renming
, p. 4640 - 4642 (2013)
A new route for preparing C33I has been ...
Investigation of CF2 carbene on the surface of activated charcoal in the synthesis of trifluoroiodomethane via vapor-phase catalytic reaction
Yang, Guang-Cheng,Lei, Shi,Pan, Ren-Ming,Quan, Heng-Dao
, p. 231 - 235 (2009)
This paper investigates the synthetic me...
Synthesis of Au(I) trifluoromethyl complexes. Oxidation to Au(III) and reductive elimination of halotrifluoromethanes
Blaya, Mara,Bautista, Delia,Gil-Rubio, Juan,Vicente, Jos
, p. 6358 - 6368 (2014)
Au(I) trifluoromethyl complexes [Au(CF3)...
Catalysts and integrated processes for producing trifluoroiodomethane
-
Page/Page column 10-12, (2020/07/07)
The present disclosure provides a proces...
ONE STEP PROCESS FOR MANUFACTURING TRIFLUOROIODOMETHANE FROM TRIFLUOROACETYL HALIDE, HYDROGEN, AND IODINE
-
Paragraph 0048-0050, (2020/08/30)
The present disclosure provides a proces...
2314-97-8 Process route
-
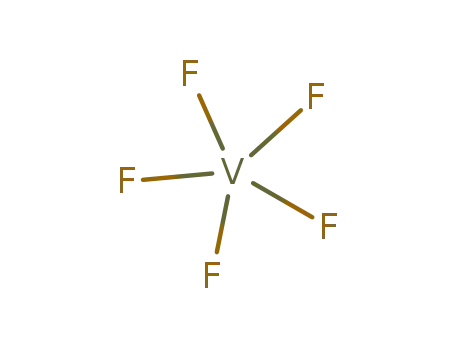
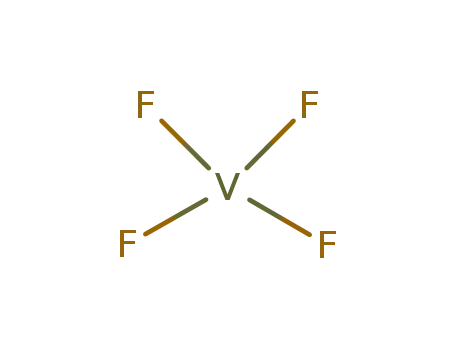
- 7783-72-4,44247-54-3
vanadium pentafluoride

-
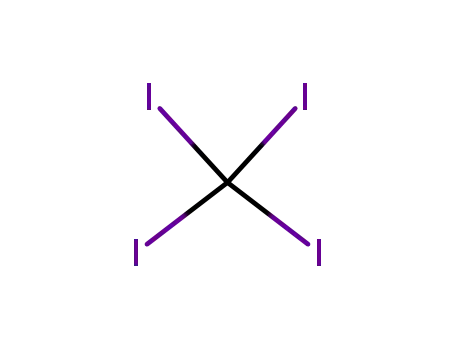
- 507-25-5
carbon tetraiodide

-
- 10049-16-8
vanadium(IV) fluoride

-
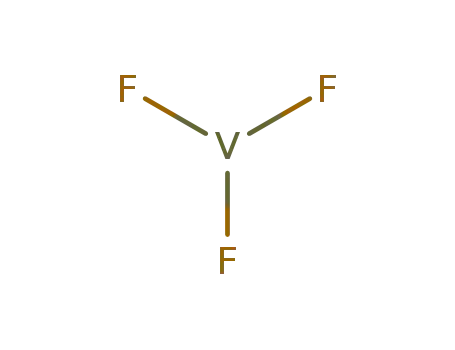
- 10049-12-4
vanadium(III) fluoride

-
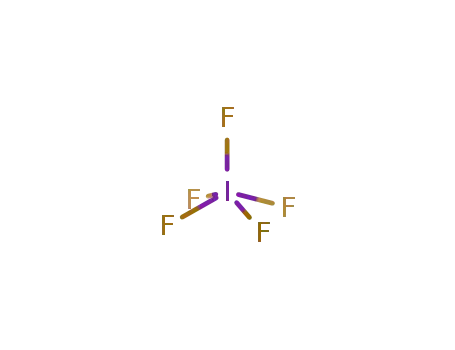
- 676353-70-1,51096-45-8,7783-66-6
iodine pentafluoride

-
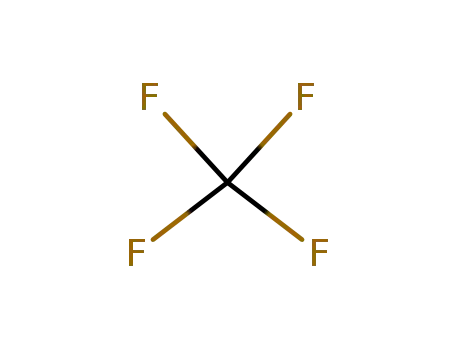
- 75-73-0
carbon tetrafluoride

-
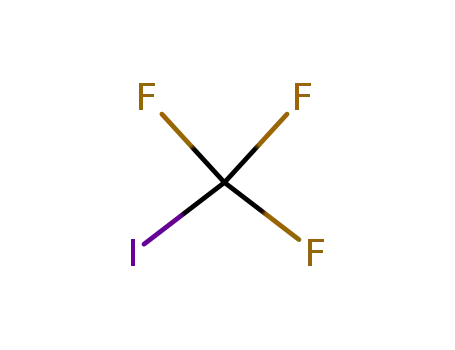
- 2314-97-8,263005-66-9
iodotrifluoromethane
| Conditions | Yield |
|---|---|
|
In neat (no solvent); after 48 h at ambiente temp.;;
|
|
|
In neat (no solvent); after 48 h at ambiente temp.;;
|
-

- 7783-72-4,44247-54-3
vanadium pentafluoride

-

- 507-25-5
carbon tetraiodide

-

- 676353-70-1,51096-45-8,7783-66-6
iodine pentafluoride

-

- 75-73-0
carbon tetrafluoride

-

- 2314-97-8,263005-66-9
iodotrifluoromethane

-
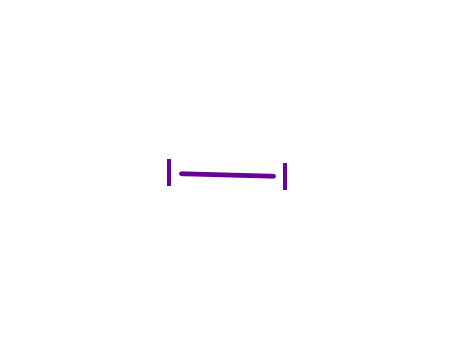
- 7553-56-2,12190-71-5,8031-47-8
iodine

-
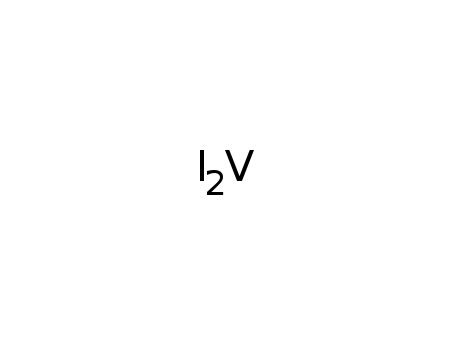
- 15513-84-5
vanadium(II) iodide
| Conditions | Yield |
|---|---|
|
In neat (no solvent); reaction of CI4 with an excess of VF5 for 48 hours; further products;;
|
|
|
In neat (no solvent); reaction of CI4 with an excess of VF5 for 48 hours; further products;;
|
2314-97-8 Upstream products
-
354-32-5
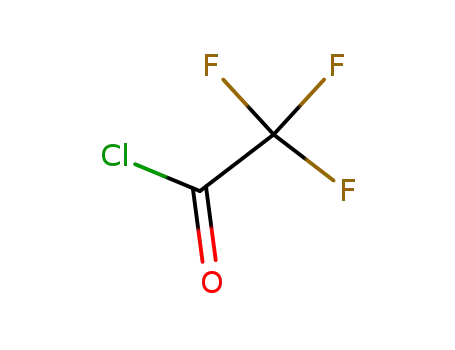
trifluoracetyl chloride
-
359-64-8
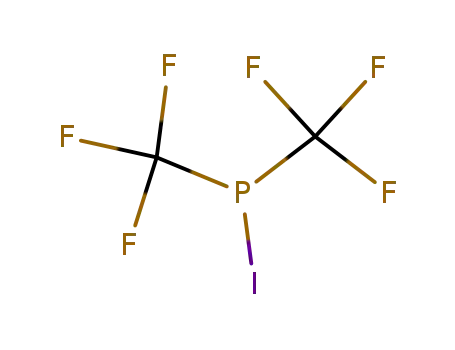
iodo-bis-trifluoromethyl-phosphine
-
507-25-5

carbon tetraiodide
-
432-02-0
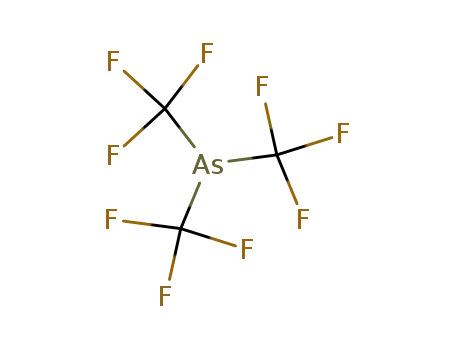
tris(trifluoromethyl)arsine
2314-97-8 Downstream products
-
540-87-4
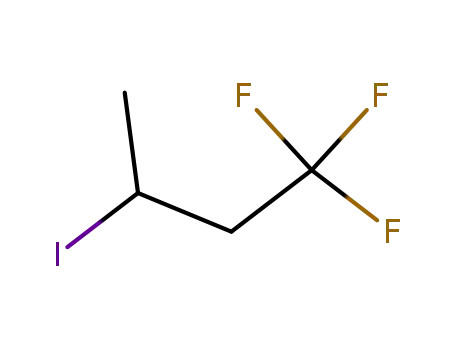
3-iodo-1,1,1-trifluorobutane
-
382-25-2
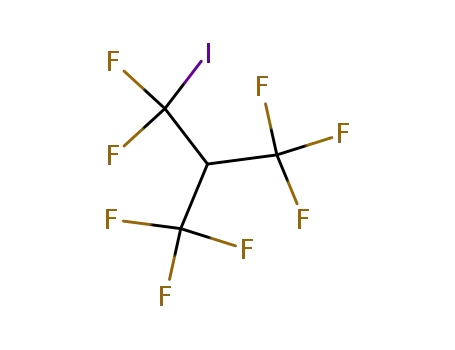
1,1,1,3,3-pentafluoro-3-iodo-2-trifluoromethyl-propane
-
75-46-7
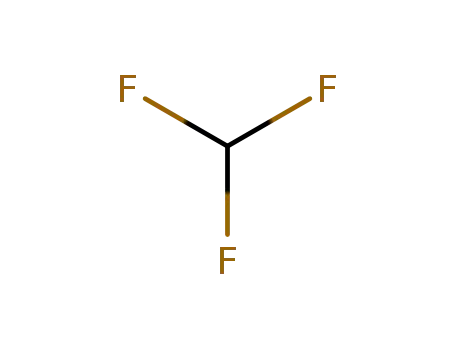
trifluoromethan
-
76-16-4
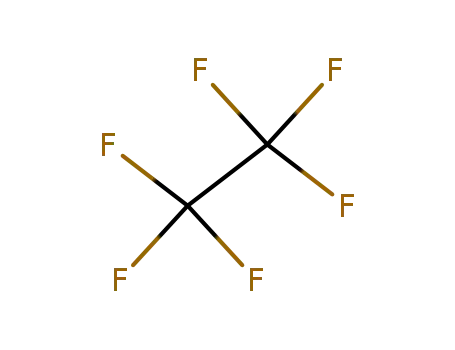
Hexafluoroethane
Relevant Products
-
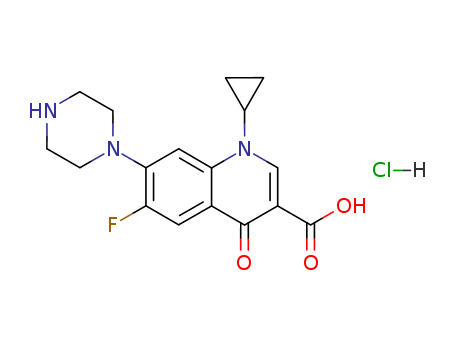
Ciprofloxacin HCl
CAS:93107-08-5
-
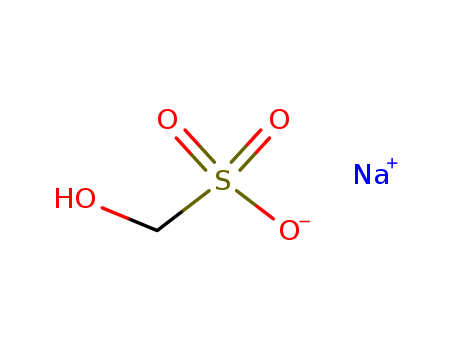
Sodium formaldehyde bisulfite
CAS:870-72-4
-
SODIUM TRIMETHYLPENTENE/MA COPOLYMER
CAS:37199-81-8