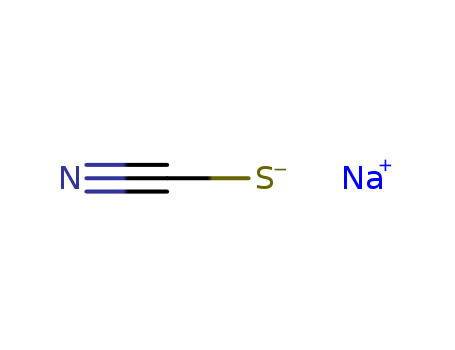
12125-02-9
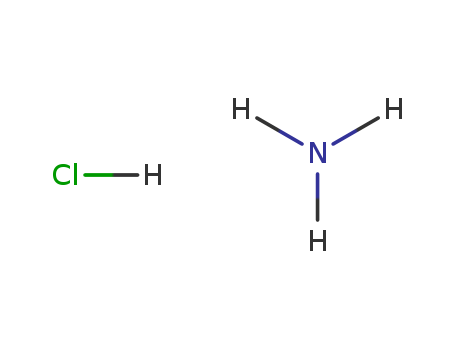
- Product Name:Ammonium chloride
- Molecular Formula:NH4Cl
- Purity:99%
- Molecular Weight:53.4915
Product Details;
CasNo: 12125-02-9
Molecular Formula: NH4Cl
Appearance: white crystalline solid
Export Quality Manufacturer Supply Ammonium chloride 12125-02-9 Safe Transportation
- Molecular Formula:NH4Cl
- Molecular Weight:53.4915
- Appearance/Colour:white crystalline solid
- Melting Point:338 °C (decomposes)
- Refractive Index:1.642
- Boiling Point:40.4 °C at 760 mmHg
- PSA:0.00000
- Density:1.5274 g/cm3
- LogP:-2.61980
- IDLH: N.D.See: IDLH INDEX
Ammonium chloride(Cas 12125-02-9) Usage
|
Chemical Description |
Ammonium chloride, K2CO3, and NaHCO3 are all chemicals used as buffers in the reaction. |
|
Who Evaluation |
Evaluation year: 1979 |
|
EXPOSURE ROUTES |
inhalation, skin and/or eye contact |
|
FIRST AID |
(See procedures) Eye:Irrigate immediately Skin:Soap wash immediately Breathing:Respiratory support |
|
Consumer Uses |
ECHA has no public registered data indicating whether or in which chemical products the substance might be used. ECHA has no public registered data on the routes by which this substance is most likely to be released to the environment. |
InChI:InChI=1/ClH.H3N/h1H;1H3
12125-02-9 Relevant articles
Room-Temperature Catalytic Reduction of Aqueous Nitrate to Ammonia with Ni Nanoparticles Immobilized on an Fe3O4@n-SiO2@h-SiO2–NH2 Support
Rai, Rohit Kumar,Tyagi, Deepika,Singh, Sanjay Kumar
, p. 2450 - 2456 (2017)
Efficient and selective catalytic reduct...
One-pot, room-temperature conversion of dinitrogen to ammonium chloride at a main-group element
Légaré, Marc-André,Bélanger-Chabot, Guillaume,Rang, Maximilian,Dewhurst, Rian D.,Krummenacher, Ivo,Bertermann, Rüdiger,Braunschweig, Holger
, p. 1076 - 1080 (2020)
The industrial reduction of dinitrogen (...
Nitrogen reduction and functionalization by a multimetallic uranium nitride complex
Falcone, Marta,Chatelain, Lucile,Scopelliti, Rosario,?ivkovi?, Ivica,Mazzanti, Marinella
, p. 332 - 335 (2017)
Molecular nitrogen (N2) is cheap and wid...
Cleavage of Dinitrogen from Forming Gas by a Titanium Molecular System under Ambient Conditions
González-Moreiras, Mariano,Mena, Miguel,Pérez-Redondo, Adrián,Yélamos, Carlos
, p. 3558 - 3561 (2017)
Simple exposure of a hexane solution of ...
Erratum: Lessons learned and lessons to be learned for developing homogeneous transition metal complexes catalyzed reduction of N2 to ammonia (Journal of Organometallic Chemistry (2014) 752 (44-58))
Sivasankar, Chinnappan,Baskaran, Sambath,Tamizmani, Masilamani,Ramakrishna, Kankanala
, p. 74 - 74 (2014)
-
Ammonia Synthesis by Hydrogenolysis of Titanium-Nitrogen Bonds Using Proton Coupled Electron Transfer
Pappas, Iraklis,Chirik, Paul J.
, p. 3498 - 3501 (2015)
The catalytic hydrogenolysis of the tita...
Dinitrogen activation upon reduction of a triiron(II) complex
Lee, Yousoon,Sloane, Forrest T.,Blondin, Genevive,Abboud, Khalil A.,Garca-Serres, Ricardo,Murray, Leslie J.
, p. 1499 - 1503 (2015)
Reaction of a trinuclear iron(II) comple...
Ligand-Based Control of Single-Site vs. Multi-Site Reactivity by a Trichromium Cluster
Bartholomew, Amymarie K.,Juda, Cristin E.,Nessralla, Jonathon N.,Lin, Benjamin,Wang, SuYin Grass,Chen, Yu-Sheng,Betley, Theodore A.
, p. 5687 - 5691 (2019)
The trichromium cluster (tbsL)Cr3(thf) (...
Cluster Supported by Redox-Active o-Phenylenediamide Ligands and Its Application toward Dinitrogen Reduction
Liang, Qiuming,Demuth, Joshua C.,Radovi?, Aleksa,Wolford, Nikki J.,Neidig, Michael L.,Song, Datong
, p. 13811 - 13820 (2021)
As prevalent cofactors in living organis...
Dinitrogen activation by dihydrogen and a PNP-ligated titanium complex
Wang, Baoli,Luo, Gen,Nishiura, Masayoshi,Hu, Shaowei,Shima, Takanori,Luo, Yi,Hou, Zhaomin
, p. 1818 - 1821 (2017)
The hydrogenolysis of the PNP-ligated ti...
Facile Dinitrogen and Dioxygen Cleavage by a Uranium(III) Complex: Cooperativity Between the Non-Innocent Ligand and the Uranium Center
Wang, Penglong,Douair, Iskander,Zhao, Yue,Wang, Shuao,Zhu, Jun,Maron, Laurent,Zhu, Congqing
, p. 473 - 479 (2021)
Activation of dinitrogen (N2, 78 %) and ...
Synthesis of calcium carbonate in trace water environments
Magnabosco, Giulia,Polishchuk, Iryna,Pokroy, Boaz,Rosenberg, Rose,C?lfen, Helmut,Falini, Giuseppe
, p. 4811 - 4814 (2017)
Calcium carbonate (CaCO3) was synthesize...
Oligo(ω-pentadecalactone) decorated magnetic nanoparticles
Razzaq, Muhammad Yasar,Behl, Marc,Frank, Ute,Koetz, Joachim,Szczerba, Wojciech,Lendlein, Andreas
, p. 9237 - 9243 (2012)
Hybrid magnetic nanoparticles (mgNP) wit...
Catalytic Dinitrogen Reduction to Ammonia at a Triamidoamine–Titanium Complex
Doyle, Laurence R.,Wooles, Ashley J.,Jenkins, Lucy C.,Tuna, Floriana,McInnes, Eric J. L.,Liddle, Stephen T.
, p. 6314 - 6318 (2018)
Catalytic reduction of N2 to NH3 by a Ti...
Direct formation of [NH4]N3 from a pentazolate salt through single-crystal to single-crystal transformation
Yang, Chen,Sun, Chengguo,Zhang, Chong,Hu, Bingcheng
, p. 144 - 147 (2018)
In the area of polynitrogen anions, the ...
In Situ Derived Bi Nanoparticles Confined in Carbon Rods as an Efficient Electrocatalyst for Ambient N2Reduction to NH3
Wang, Fengyi,Zhang, Longcheng,Wang, Ting,Zhang, Fang,Liu, Qian,Zhao, Haitao,Zheng, Baozhan,Du, Juan,Sun, Xuping
, p. 7584 - 7589 (2021)
Electrocatalytic N2 reduction is deemed ...
Dinitrogen Activation and Hydrogenation by C5Me4SiMe3-Ligated Di- And Trinuclear Chromium Hydride Complexes
Shima, Takanori,Yang, Jimin,Luo, Gen,Luo, Yi,Hou, Zhaomin
, p. 9007 - 9016 (2020)
Activation of dinitrogen (N2) by well-de...
Evaluating Molecular Cobalt Complexes for the Conversion of N2 to NH3
Del Castillo, Trevor J.,Thompson, Niklas B.,Suess, Daniel L. M.,Ung, Ga?l,Peters, Jonas C.
, p. 9256 - 9262 (2015)
Well-defined molecular catalysts for the...
Infrared Spectroscopic Study of the Cryogenic Thin Film and Matrix-Isolated Complexes of TiCl4 with NH3 and (CH3)3N
Everhart, Jennifer B.,Ault, Bruce S.
, p. 4379 - 4384 (1995)
The matrix isolation technique and infra...
Degradation of the beta-blocker propranolol by electrochemical advanced oxidation processes based on Fenton's reaction chemistry using a boron-doped diamond anode
Isarain-Chávez, Eloy,Rodríguez, Rosa María,Garrido, José Antonio,Arias, Conchita,Centellas, Francesc,Cabot, Pere Lluís,Brillas, Enric
, p. 215 - 221 (2010)
The electro-Fenton (EF) and photoelectro...
Dinitrogen cleavage by a heterometallic cluster featuring multiple uranium-rhodium bonds
Xin, Xiaoqing,Douair, Iskander,Zhao, Yue,Wang, Shuao,Maron, Laurent,Zhu, Congqing
, p. 15004 - 15011 (2020)
Reduction of dinitrogen (N2) is a major ...
Niobium-nitrides derived from nitrogen splitting
Searles, Keith,Carroll, Patrick J.,Chen, Chun-Hsing,Pink, Maren,Mindiola, Daniel J.
, p. 3526 - 3528 (2015)
The easy-to-prepare Nb(v) aryloxide comp...
CLUSTER BEAM CHEMISTRY: ADDUCTS OF HYDROGEN HALIDES WITH AMMONIA CLUSTERS
Cheung, Jeffery T.,Dixon, David A.,Herschbach, Dudley R.
, p. 2536 - 2541 (1988)
A molecular beam of ammonia clusters (NH...
Preparation of carboxyl group functionalized magnetite nanoparticles with high magnetization
Hung, Nguyen Quang,Cuong, Nguyen Viet,Van Thang, Tran,Nguyen, Hoang Thi Kieu
, p. 3815 - 3819 (2014)
A new route of the emulsifier-free emuls...
Efficient catalytic conversion of dinitrogen to N(SiMe3)3 Using a homogeneous mononuclear cobalt complex
Suzuki, Tatsuya,Fujimoto, Keisuke,Takemoto, Yoshiyuki,Wasada-Tsutsui, Yuko,Ozawa, Tomohiro,Inomata, Tomohiko,Fryzuk, Michael D.,Masuda, Hideki
, p. 3011 - 3015 (2018)
Incorporation of the tridentate phosphin...
Amidinate Supporting Ligands Influence Molecularity in Formation of Uranium Nitrides
Arnold, John,Booth, Corwin H.,Boreen, Michael A.,Hohloch, Stephan,Lohrey, Trevor D.,Minasian, Stefan G.,Moreau, Liane M.,Ouellette, Erik T.,Qiao, Yusen,Settineri, Nicholas S.,Straub, Mark D.
, p. 6672 - 6679 (2021)
Uranium nitride complexes are attractive...
Tripodal P3XFe-N2Complexes (X = B, Al, Ga): Effect of the Apical Atom on Bonding, Electronic Structure, and Catalytic N2-to-NH3Conversion
Fajardo, Javier,Peters, Jonas C.
supporting information, p. 1220 - 1227 (2021/02/05)
Terminal dinitrogen complexes of iron li...
Stepwise Reduction of Dinitrogen by a Uranium-Potassium Complex Yielding a U(VI)/U(IV) Tetranitride Cluster
?ivkovi?, Ivica,Barluzzi, Luciano,Douair, Iskander,Fadaei-Tirani, Farzaneh,Jori, Nadir,Maron, Laurent,Mazzanti, Marinella
supporting information, p. 11225 - 11234 (2021/08/03)
Multimetallic cooperativity is believed ...
12125-02-9 Process route
-
-
C47H42N5O3Ti

-
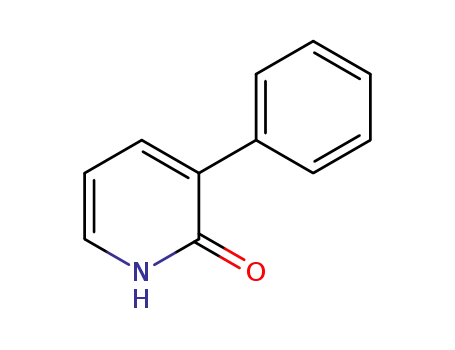
-
24228-13-5
2-hydroxy-3-phenylpyridine

-

-
780-25-6
N-benzylidene benzylamine

-
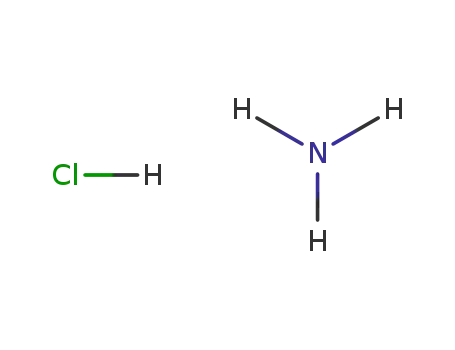
-
12125-02-9
ammonium chloride

-
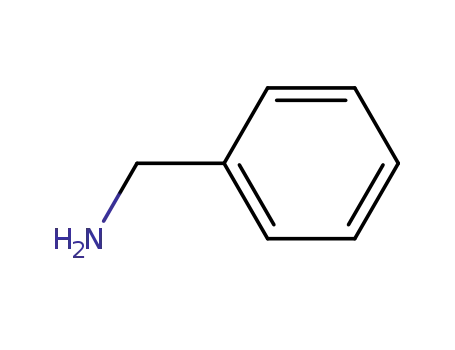
-
100-46-9
benzylamine
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride;
In
1,4-dioxane;
Inert atmosphere;
Glovebox;
Schlenk technique;
|
-
-
C2H5NO2*H3N*ClH

-

-
12125-02-9
ammonium chloride

-
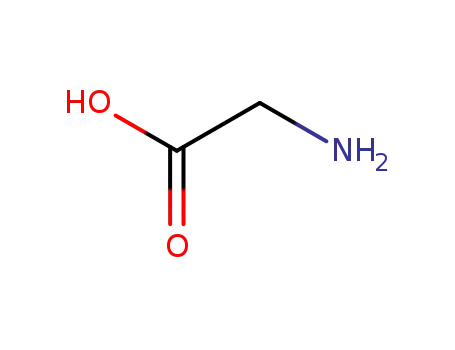
-
56-40-6,18875-39-3,25718-94-9
glycine
| Conditions | Yield |
|---|---|
|
In
methanol; ethylene glycol;
at 65 ℃;
Solvent;
|
39.13 g |
12125-02-9 Upstream products
-
79-37-8
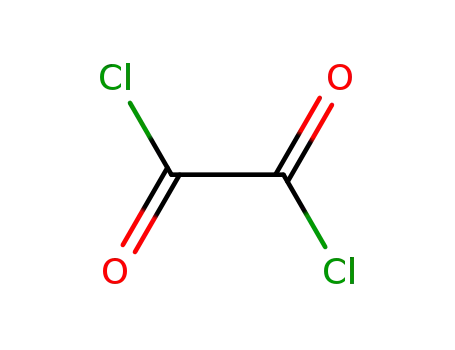
oxalyl dichloride
-
172733-07-2
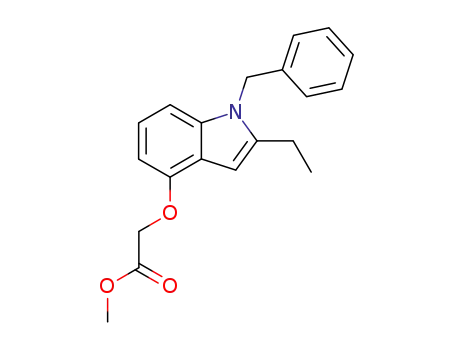
2-[[2-Ethyl-1-(phenylmethyl)-1H-indol-4-yl]oxy]acetic acid methyl ester
-
1467-16-9
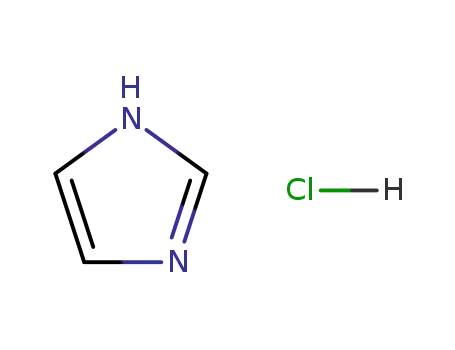
Imidazole hydrochloride
-
7647-01-0
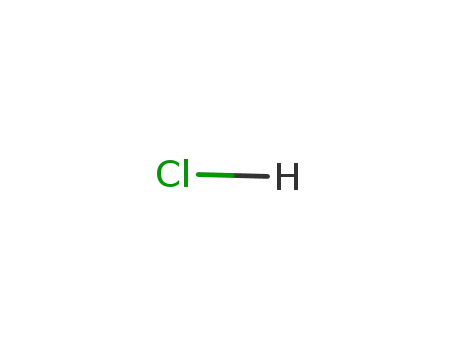
hydrogenchloride
12125-02-9 Downstream products
-
170794-70-4
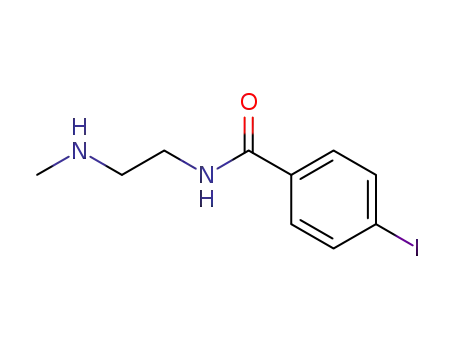
4-iodo-N-(2-methylaminoethyl)benzamide
-
439124-52-4
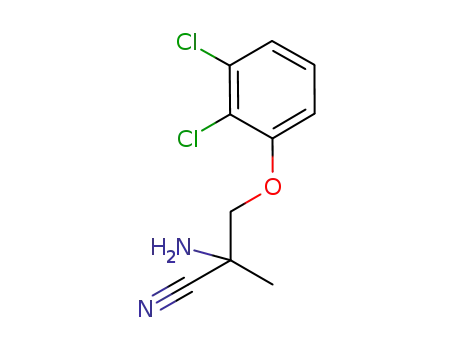
2-amino-3-(2,3-dichlorophenoxy)-2-methylpropionitrile
-
247199-97-9
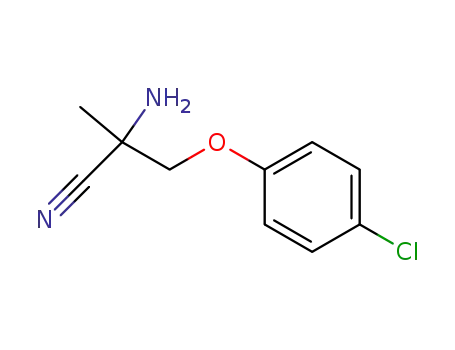
2-amino-2-methyl-3-(4-chlorophenoxy)propanenitrile
-
710350-60-0
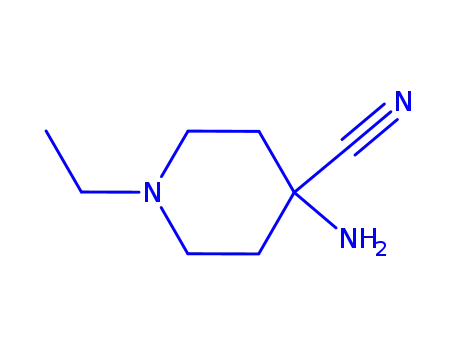
4-amino-4-cyano-1-ethylpiperidine
Relevant Products
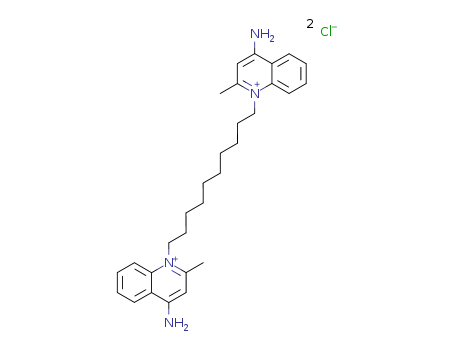
-
Dequalinium chloride
CAS:522-51-0
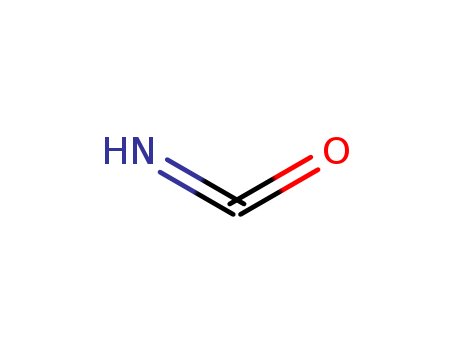
-
ISOCYANIC ACID
CAS:75-13-8
-
Sodium thiocyanate
CAS:540-72-7