522-51-0
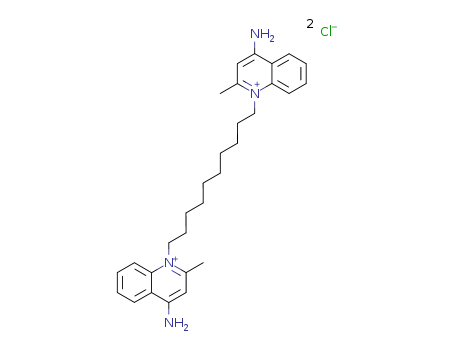
- Product Name:Dequalinium chloride
- Molecular Formula:C30H40Cl2N4
- Purity:99%
- Molecular Weight:527.58
Product Details;
CasNo: 522-51-0
Molecular Formula: C30H40Cl2N4
Chinese Manufacturer Supply Reliable Quality 522-51-0 Dequalinium chloride with Competitive Price
- Molecular Formula:C30H40Cl2N4
- Molecular Weight:527.58
- Melting Point:≥300 °C(lit.)
- PSA:59.80000
- LogP:1.34060
Dequalinium chloride(Cas 522-51-0) Usage
|
Description |
Dequalinium chloride is a quaternary ammonium cation and bolaamphiphile, often found as the dichloride salt. As a versatile antiseptic and disinfectant, it has been employed for over 60 years to treat local infections. Dequalinium chloride is a standard medication for bacterial vaginosis and a key ingredient in sore-throat lozenges. It is also known by various salts such as bromide, iodide, acetate, and undecenoate. |
|
Chemical Properties |
White or yellowish-white powder, hygroscopic. |
|
Originator |
Dequsan,Sante |
|
Uses |
MitoBloCK-12, a compound containing dequalinium chloride, has been discovered to attenuate mitochondrial protein import, with applications in treating primary hyperoxaluria 1. In addition to its antimicrobial activity, dequalinium chloride inhibits apamin-sensitive K+ channels and induces apoptosis by inhibiting XIAP. This compound is described as a white or yellowish-white powder with hygroscopic properties. |
|
Definition |
ChEBI: An organic chloride salt that is the dichloride salt of dequalinium. |
|
Manufacturing Process |
Manufacturing processes involve the refluxing of 4-aminoquinaldine and decamethylene diiodide, resulting in the formation of 1,1'-decamethylenebis(4-aminoquinaldinium chloride). Dequalinium chloride is available under brand names such as Decabis, Dequacaine, and Grocreme, and it is recognized for its therapeutic functions as an antiseptic and antifungal agent. However, skin reactions, including necrotic lesions, have been reported, leading to its use as a mouth and throat disinfectant in various countries. |
|
Brand name |
Decabis;Dequacaine;Dequafungan;Dequin;Faringina;Gargilon;Grocreme;Labosept;Maltyl;Phylletten;Soor-gel;Sorot;Tetesept. |
|
Therapeutic Function |
Antiseptic, Antifungal |
|
World Health Organization (WHO) |
Skin reactions to dequalinium chloride, including necrotic lesions, have been reported. It remains available as a mouth and throat disinfectant in many countries. |
InChI:InChI=1/C30H38N4.2ClH/c1-23-21-27(31)25-15-9-11-17-29(25)33(23)19-13-7-5-3-4-6-8-14-20-34-24(2)22-28(32)26-16-10-12-18-30(26)34;;/h9-12,15-18,21-22,31-32H,3-8,13-14,19-20H2,1-2H3;2*1H
522-51-0 Relevant articles
Medicinal applications and molecular targets of dequalinium chloride
Christian Bailly
, Biochemical Pharmacology Volume 186, April 2021, 114467
At the bacterial level, Dequalinium chloride interacts with different multidrug transporters (QacR, AcrB, EmrE) and with the transcriptional regulator RamR. Other proteins implicated in the antiviral (MPER domain of gp41 HIV-1) and antiparasitic (chitinase A from Vibrio harveyi) activities have been identified. Dequalinium chloride also targets α -synuclein oligomers to restrict protofibrils formation implicated in some neurodegenerative disorders.
Spectrophotometric determination of dequalinium chloride in pharmaceutical preparations
C. P. Leung and S. Y. Kwan
, Analyst, Issue 1235, 1979
Dequalinium chloride [decamethylenebis(4-aminoquinaldinium chloride)] is a quaternary ammonium compound with antibacterial and antifungal properties. It is commionly used in the form of lozenges and paints. for the treatment of infections of the mouth and throat. The compound is described in the British Pharmacopoeial and the method of assay therein is based on non-aqueous titration with perchlorie acid in 1 ,4-dioxan.
Preparation method of dequalinium chloride and its analogues
-
Paragraph 0063; 0092-0097, (2017/07/20)
The invention provides a preparation met...
522-51-0 Process route
-
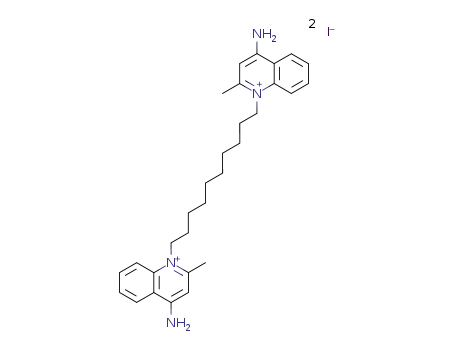
-
Dequalinium iodide

-
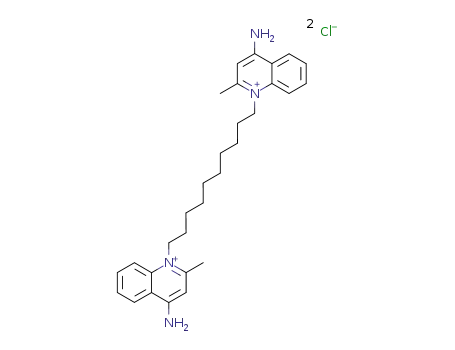
- 522-51-0
dequalinium chloride
| Conditions | Yield |
|---|---|
|
Dequalinium iodide; With hydrogenchloride; In water; at 60 - 80 ℃; for 1h; Industrial scale;
With dihydrogen peroxide; In water; at 50 ℃; for 1h; Reagent/catalyst; Temperature; Industrial scale;
|
89% |
|
Dequalinium iodide; With hydrogenchloride; In water; at 80 - 90 ℃; for 1h; Industrial scale;
With chlorine; sodium hydroxide; In water; at 50 ℃; for 1h; Temperature; Solvent; Reagent/catalyst; Industrial scale;
|
79% |
|
With hydrogenchloride; In water; at 100 ℃; for 1h; Temperature;
|
-
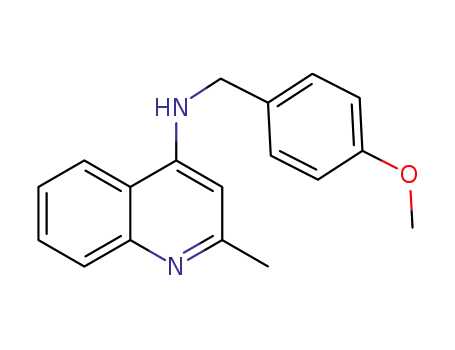
-
2-methyl-4-(N-(4-methoxy)benzylamine)quinolin

-

- 522-51-0
dequalinium chloride
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 3 steps
1: trifluoroacetic acid / 2.5 h / 80 °C
2: sulfolane / 12 h / 80 °C
3: hydrogenchloride / water / 1 h / 100 °C
With hydrogenchloride; trifluoroacetic acid; In sulfolane; water;
|
522-51-0 Upstream products
-
4295-06-1
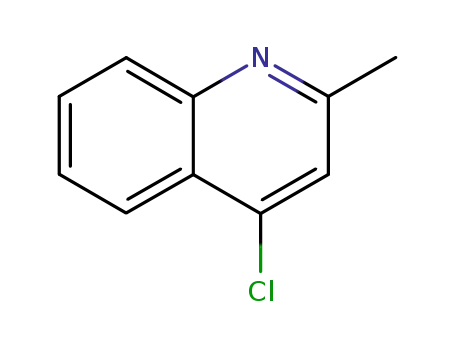
4-chloro-2-methylquinoline
Relevant Products
-
Ciprofloxacin HCl
CAS:93107-08-5
-
MITRAGYNINE PICRATE
CAS:4098-40-2