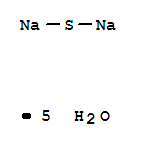
13601-19-9
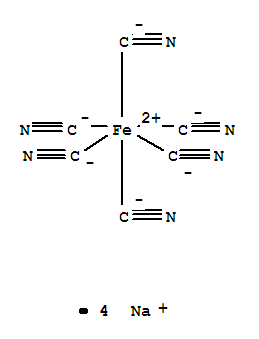
- Product Name:Sodium ferrocyanide
- Molecular Formula:Na4Fe.(CN)6
- Purity:99%
- Molecular Weight:484.06
Product Details;
CasNo: 13601-19-9
Molecular Formula: Na4Fe.(CN)6
Appearance: Odorless yellow solid
Buy High Grade Top Purity 99% Sodium ferrocyanide 13601-19-9 Safe Transportation
- Molecular Formula:Na4Fe.(CN)6
- Molecular Weight:484.06
- Appearance/Colour:Odorless yellow solid
- Melting Point:82°C -10H?O
- Boiling Point:25.7oC at 760 mmHg
- PSA:142.74000
- Density:1.458 g/cm3
- LogP:0.09818
Sodium ferrocyanide(Cas 13601-19-9) Usage
|
Description |
Sodium ferrocyanide is an odorless yellow solid and the sodium salt of the coordination compound [Fe(CN)6]4?. Also known as yellow prussiate of soda in its hydrous form (Na4Fe(CN)6 ? 10H2O), it is a yellow crystalline solid with solubility in water and insolubility in alcohol. The yellow color is attributed to the ferrocyanide anion. Despite containing cyanide ligands, sodium ferrocyanide has low toxicity, with an acceptable daily intake of 0–0.025 mg/kg body weight. It is considered less toxic than many other cyanide salts. |
|
Production |
Sodium ferrocyanide is produced industrially from hydrogen cyanide, ferrous chloride, and calcium hydroxide, the combination of which affords Ca2[Fe(CN)6] ? 11H2O. A solution of this salt is then treated with sodium salts to precipitate the mixed calcium-sodium salt CaNa2[Fe(CN)6], which in turn is treated with sodium carbonate to give the tetrasodium salt. |
|
Storage |
Hygroscopic, -20°C Freezer, Under inert atmosphere |
|
Reactivity |
Sodium ferrocyanide is a coordination compound of iron. The cyanide ligands are tightly bound to the iron, so it is not as toxic as simple inorganic cyanide salts. However, this compound can release hydrogen cyanide gas upon reaction with acids. |
|
appearance |
Sodium ferrocyanide is a yellow crystalline salt Na4Fe(CN)6 similar to potassium ferrocyanide and used in making iron blue pigments, blueprint paper, and dyes — Called also yellow prussiate of soda |
|
Chemical Properties |
solid |
|
Uses |
Toxicity: Sodium ferrocyanide is less toxic than many other cyanide salts, posing a low risk of releasing free cyanide. Hydrogen Cyanide Production: While generally low in toxicity, the addition of an acid to sodium ferrocyanide can produce hydrogen cyanide gas, which is toxic. Sodium Ion Batteries: Sodium hexacyanoferrate(II) decahydrate, a form of sodium ferrocyanide, is being investigated as a potential cathode material for sodium ion batteries. Manufacture: Used in the manufacture of sodium ferricyanide, blue pigments, and blueprint paper. |
|
Air & Water Reactions |
Water soluble. |
|
Reactivity Profile |
Sodium ferrocyanide has weak oxidizing or reducing powers. Redox reactions can however still occur. The majority of compounds in this class are slightly soluble or insoluble in water. If soluble in water, then the solutions are usually neither strongly acidic nor strongly basic. These compounds are not water-reactive. |
|
Health Hazard |
None recorded. |
|
Flammability and Explosibility |
Nonflammable |
|
Safety Profile |
When heated to decomposition emits toxic fumes of CN-. |
|
Purification Methods |
Crystallise it from hot water (0.7mL/g), until free of ferricyanide as shown by the absence of formation of Prussian Blue colour with ferrous sulfate solution. |
|
Who Evaluation |
Evaluation year: 1974 |
InChI:InChI=1/12CN.2Fe.4Na/c12*1-2;;;;;;/q;;;;;;;;;;;;2*-2;4*+1/r2C6FeN6.4Na/c2*8-1-7(2-9,3-10,4-11,5-12)6-13;;;;/q2*-2;4*+1
Relevant Products
-
SODIUM SULFIDE PENTAHYDRATE
CAS:1313-83-3
-
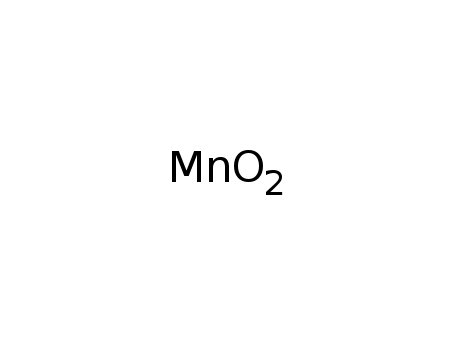
Manganese dioxide
CAS:1313-13-9
-
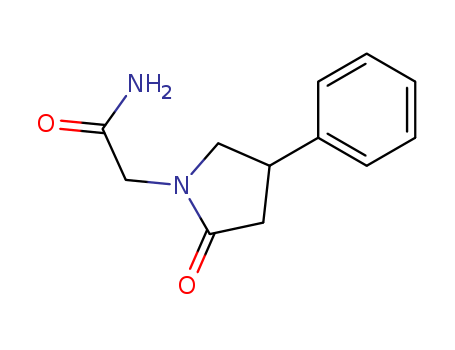
4-Phenyl-2-pyrrolidone-1-acetamide
CAS:77472-70-9