77472-70-9
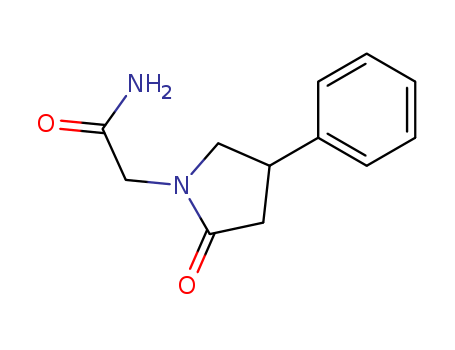
- Product Name:4-Phenyl-2-pyrrolidone-1-acetamide
- Molecular Formula:C12H14N2O2
- Purity:99%
- Molecular Weight:218.255
Product Details;
CasNo: 77472-70-9
Molecular Formula: C12H14N2O2
Quality Manufacturer Supply 99% Pure 4-Phenyl-2-pyrrolidone-1-acetamide 77472-70-9 Cheapest Price
- Molecular Formula:C12H14N2O2
- Molecular Weight:218.255
- Vapor Pressure:1.3E-09mmHg at 25°C
- Melting Point:129.5-130.5 °C(Solv: ethyl acetate (141-78-6))
- Refractive Index:1.579
- Boiling Point:486.4 °C at 760 mmHg
- PKA:15.67±0.40(Predicted)
- Flash Point:247.9 °C
- PSA:64.39000
- Density:1.22 g/cm3
- LogP:1.57540
4-Phenyl-2-pyrrolidone-1-acetamide(Cas 77472-70-9) Usage
|
Description |
4-Phenyl-2-pyrrolidone-1-acetamide (also named as Phenylpiracetam, Fonturacetam) is a phenylated analog of the drug piracetam, which was developed in 1983 in Russia. It is now available as a prescription drug. It has antiamnesic, anticonvulsant and anxiolytic properties. It is used as a general stimulant or to increase tolerance to extreme temperatures and stress. |
|
Uses |
Carphedone is a GABA derivative that exhibited apparent immunocorrection properties during immunosuppression induced by cyclophosphamide were studied. |
InChI:InChI=1/C12H14N2O2/c13-11(15)8-14-7-10(6-12(14)16)9-4-2-1-3-5-9/h1-5,10H,6-8H2,(H2,13,15)
77472-70-9 Relevant articles
Synthesis of substituted 2-(2-oxopyrrolidin-1-yl)acetamides
Kavina,Sizov,Yakovlev
, p. 873 - 878 (2017/08/02)
The reaction of chloroacetamide with 2 e...
Method for preparing phenyl piracetam
-
Paragraph 0075; 0076; 0077; 0078; 0079; 0080-0088, (2017/05/09)
The invention discloses a method for pre...
SIMPLE METHODS FOR THE N-ALKYLATION OF LACTAMS
Kramarova, E. P.,Shipov, A. G.,Orlova, N. A.,Artamkina, O. B.,Belavin, I. Yu.,Baukov, Yu. I.
, p. 970 - 979 (2007/10/02)
-
THE SYNTHESIS AND ANTISPASMODIC ACTIVITY OF 4-PHENYLPYRROLIDONE-2-ACETAMIDES
Glozman, O. M.,Morozov, I. S.,Zhmurenko, L. A.,Zagorevskii, V. A.
, p. 776 - 780 (2007/10/02)
-
77472-70-9 Process route
-
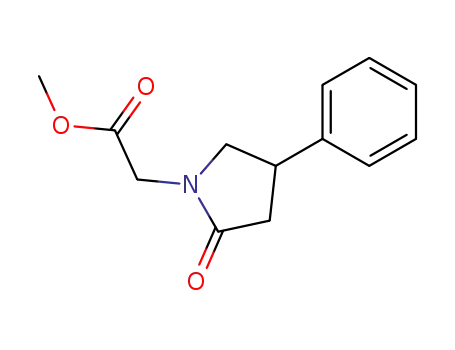
- 68497-63-2
methyl-2-oxo-4-phenylpyrrolidine-1-acetate

-
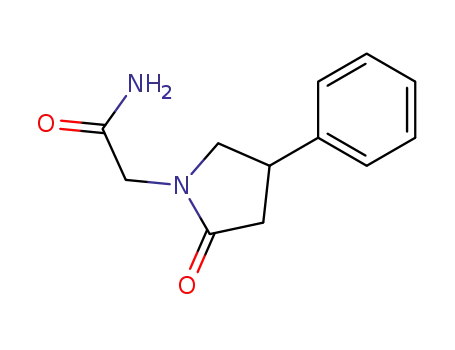
- 77472-70-9
(RS)-2-(2-oxo-4-phenylpyrrolidin-1-yl)acetamide
| Conditions | Yield |
|---|---|
|
With ammonium hydroxide; for 2h;
|
81% |
|
With ammonia; In methanol; at 20 - 30 ℃; for 12h; Concentration; Temperature; Solvent;
|
81% |
-
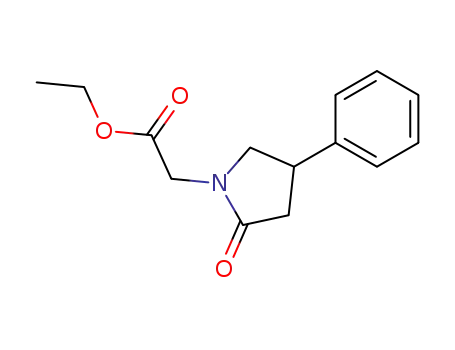
- 70291-40-6
2-oxo-4-phenyl-1-pyrrolidineacetic acid, ethyl ester

-

- 77472-70-9
(RS)-2-(2-oxo-4-phenylpyrrolidin-1-yl)acetamide
| Conditions | Yield |
|---|---|
|
With ammonia; In methanol; at 45 - 50 ℃; for 5h;
|
100% |
|
With ammonium hydroxide; for 2h;
|
64% |
77472-70-9 Upstream products
-
68497-63-2

methyl-2-oxo-4-phenylpyrrolidine-1-acetate
-
70291-40-6

2-oxo-4-phenyl-1-pyrrolidineacetic acid, ethyl ester
-
1198-97-6
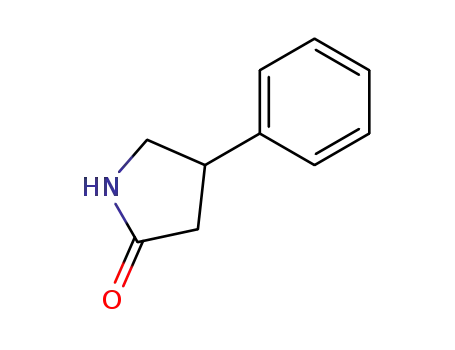
4-phenylpyrrolidin-2-one
-
106869-48-1
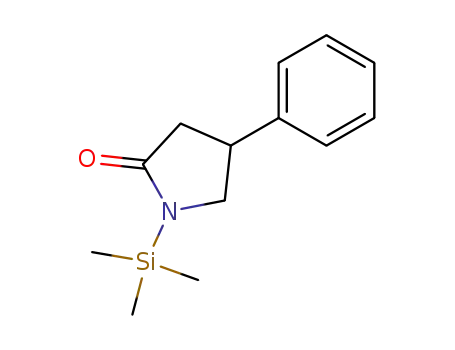
4-phenyl-1-(trimethylsilyl)-2-pyrrolidinone
77472-70-9 Downstream products
-
1613318-02-7
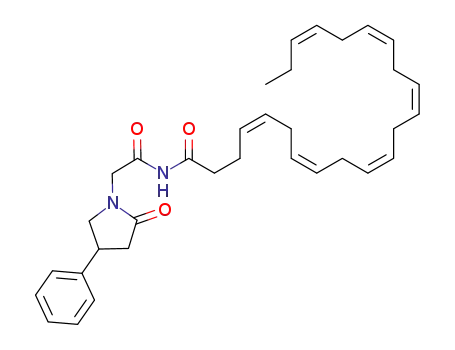
C34H44N2O3
Relevant Products
-
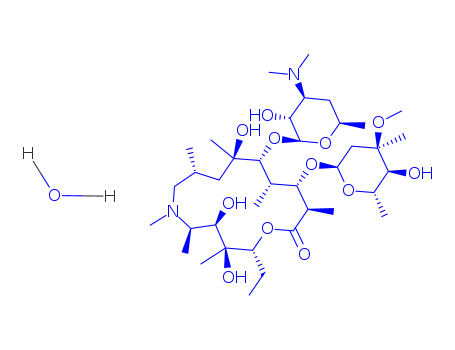
Azithromycin dihydrate
CAS:117772-70-0
-
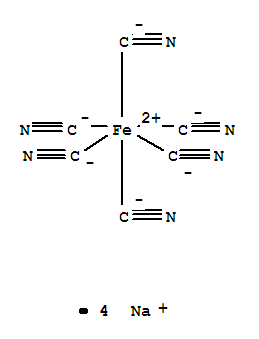
Sodium ferrocyanide
CAS:13601-19-9
-
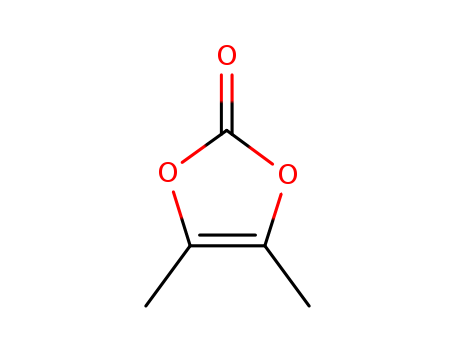
4,5-Dimethyl-1,3-dioxol-2-one
CAS:37830-90-3