3445-11-2
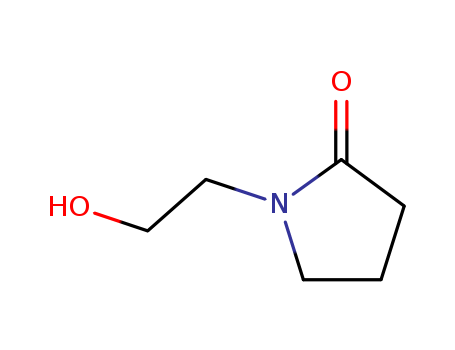
- Product Name:N-(2-Hydroxyethyl)-2-pyrrolidone
- Molecular Formula:C6H11 N O2
- Purity:99%
- Molecular Weight:129.159
Product Details;
CasNo: 3445-11-2
Molecular Formula: C6H11 N O2
Appearance: deep brown to yellow liquid
Buy Quality Hot Sale N-(2-Hydroxyethyl)-2-pyrrolidone 3445-11-2 Competitive Price
- Molecular Formula:C6H11 N O2
- Molecular Weight:129.159
- Appearance/Colour:deep brown to yellow liquid
- Vapor Pressure:0.000152mmHg at 25°C
- Melting Point:19-21°C
- Refractive Index:n20/D 1.496(lit.)
- Boiling Point:140-142 ºC (3 mmHg)
- PKA:14.49±0.10(Predicted)
- Flash Point:100 ºC
- PSA:40.54000
- Density:1.143
- LogP:-0.46100
N-(2-Hydroxyethyl)-2-pyrrolidone(Cas 3445-11-2) Usage
|
Chemical Properties |
deep brown to yellow liquid |
|
Uses |
1-(2-Hydroxyethyl)-2-pyrrolidone can be used as co-solvent for agro, coating and inkjet formulations as well as a solvent for electronics processing. |
|
Synthesis Reference(s) |
Journal of the American Chemical Society, 74, p. 4959, 1952 DOI: 10.1021/ja01139a518 |
|
Flammability and Explosibility |
Notclassified |
InChI:InChI=1/C6H11NO2/c8-5-4-7-3-1-2-6(7)9/h8H,1-5H2
3445-11-2 Relevant articles
A biodegradable transdermal penetration enhancer based on N-(2-hydroxyethyl)-2-pyrrolidone I. Synthesis and characterization
W.J. Lambert a b, R.J. Kudla b, J.M. Holland c, J.T. Curry c
, International Journal of Pharmaceutics Volume 95, Issues 1–3, 30 June 1993, Pages 181-192
In the present report, model fatty acid esters of N-(2-hydroxyethyl)-2-pyrrolidone were synthesized in order to test this approach. It was found that a 2 order of magnitude increase in permeability for hydrocortisone through mouse skin could be achieved in vitro with these enhancers.
Correlating the Synthesis, Structure, and Catalytic Performance of Pt-Re/TiO2for the Aqueous-Phase Hydrogenation of Carboxylic Acid Derivatives
Haus, Moritz O.,Meledin, Alexander,Leiting, Sebastian,Louven, Yannik,Roubicek, Nico C.,Moos, Sven,Weidenthaler, Claudia,Weirich, Thomas E.,Palkovits, Regina
, p. 5119 - 5134 (2021/05/10)
Pt-Re bimetallic catalysts have many app...
Extending the chemical product tree: A novel value chain for the production of: N -vinyl-2-pyrrolidones from biogenic acids
Haus, Moritz Otto,Louven, Yannik,Palkovits, Regina
, p. 6268 - 6276 (2019/12/03)
The sustainable production of polymers f...
Method for Producing Bio-Based Homoserine Lactone and Bio-Based Organic Acid from O-Acyl Homoserine Produced by Microorganisms
-
Paragraph 0232; 0233; 0234; 0235, (2014/10/16)
The present invention relates to a metho...
3445-11-2 Process route
-
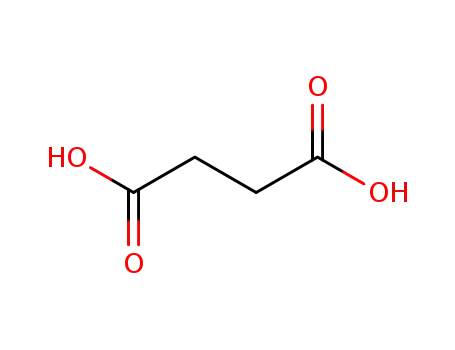
- 110-15-6
succinic acid

-
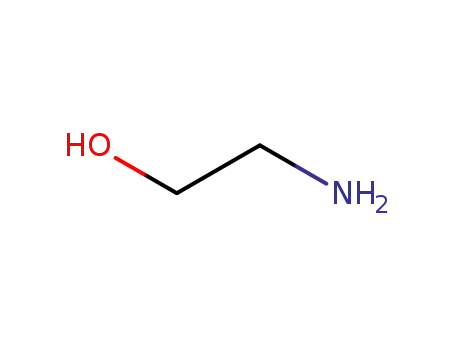
- 141-43-5
ethanolamine

-
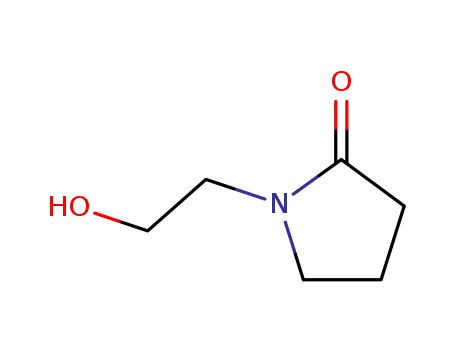
- 3445-11-2
1-(2-hydroxyethyl)-2-pyrrolidinone

-
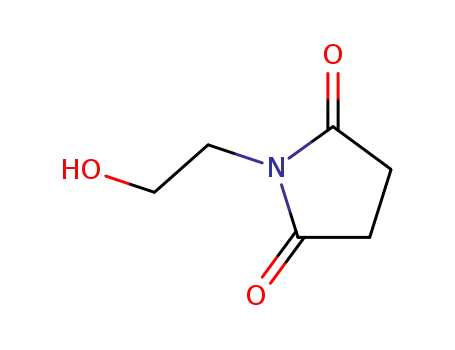
- 18190-44-8
1-(2-Hydroxy-ethyl)-pyrrolidine-2,5-dione

-
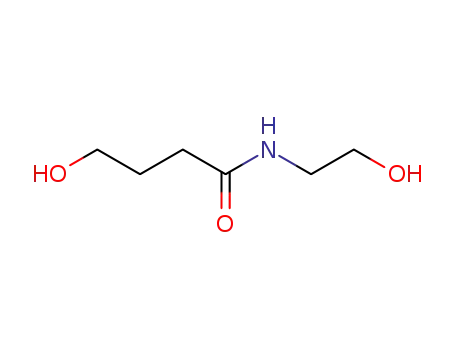
- 66857-17-8
N-(2-hydroxyethyl)-γ-hydroxybutyramide
| Conditions | Yield |
|---|---|
|
With 5% active carbon-supported ruthenium; hydrogen; In water; at 150 ℃; for 6h; under 112511 Torr; Temperature; chemoselective reaction; Catalytic behavior; Green chemistry;
|
-
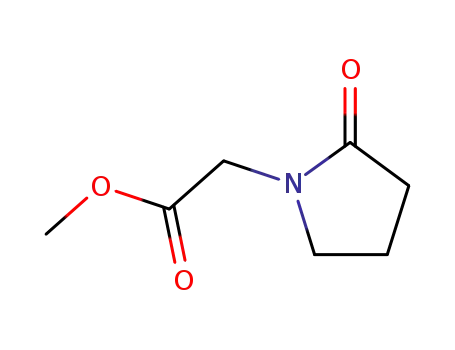
- 59776-88-4
methyl-2-oxopyrrolidine-1-acetate

-

- 3445-11-2
1-(2-hydroxyethyl)-2-pyrrolidinone
| Conditions | Yield |
|---|---|
|
With Cp*RuCl(2-C5H4CH2NH2); hydrogen; sodium methylate; In isopropyl alcohol; at 100 ℃; for 23h; under 37503.8 Torr; Autoclave;
|
85% |
|
With hydrogen; In 1,2-dimethoxyethane; at 70 ℃; for 48h; under 22502.3 Torr; chemoselective reaction; Autoclave; Molecular sieve;
|
3445-11-2 Upstream products
-
75-21-8
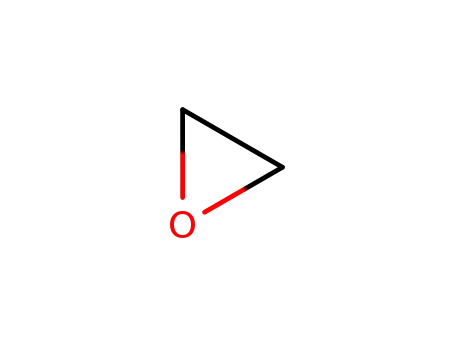
oxirane
-
123-75-1
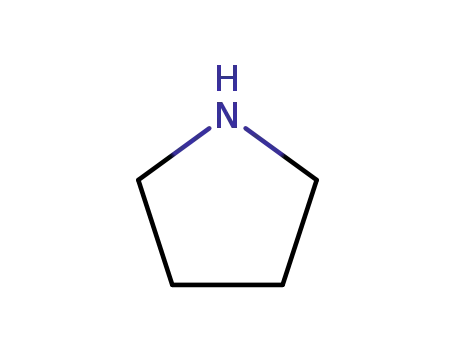
pyrrolidine
-
616-45-5
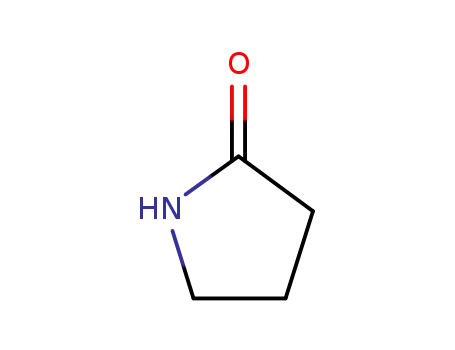
2-pyrrolidinon
-
96-48-0
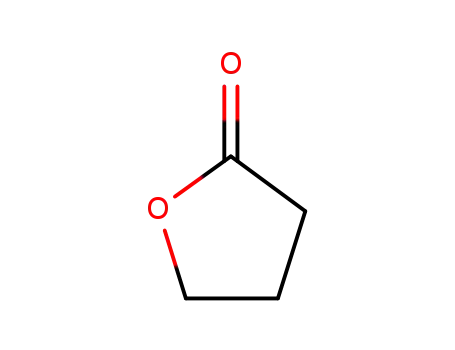
4-butanolide
3445-11-2 Downstream products
-
3541-31-9
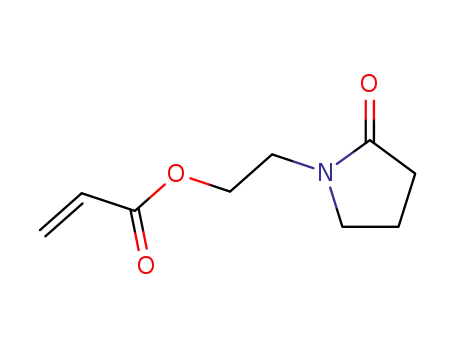
N-<2-Acryloyloxy-aethyl>-pyrrolidinon
-
86366-53-2
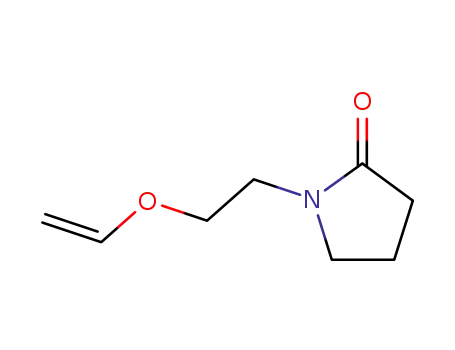
1-(2-Vinyloxy-ethyl)-pyrrolid-2-on
-
88-12-0
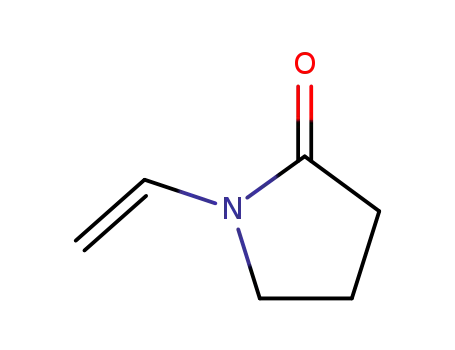
1-ethenyl-2-pyrrolidinone
-
51333-90-5
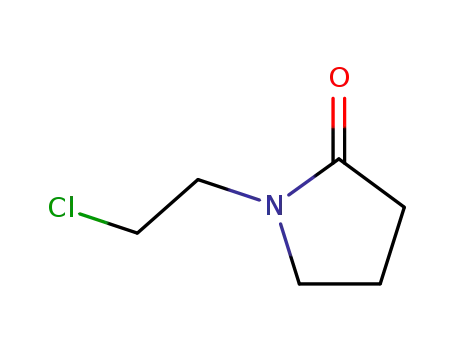
1-(2-chloro-ethyl)-pyrrolidin-2-one
Relevant Products
-
Azithromycin dihydrate
CAS:117772-70-0
-
(GLY14)-HUMANIN (HUMAN)
CAS:330936-70-4
-
Oxiracetam
CAS:62613-82-5