62613-82-5
- Product Name:Oxiracetam
- Molecular Formula:C6H10N2O3
- Purity:99%
- Molecular Weight:158.157
Product Details;
CasNo: 62613-82-5
Molecular Formula: C6H10N2O3
Appearance: white crystalline powder
Buy High Quality Reliable Quality Oxiracetam 62613-82-5 Cheapest Price
- Molecular Formula:C6H10N2O3
- Molecular Weight:158.157
- Appearance/Colour:white crystalline powder
- Vapor Pressure:7.39E-12mmHg at 25°C
- Melting Point:165-168 °C
- Refractive Index:1.57
- Boiling Point:494.6 °C at 760 mmHg
- PKA:13.76±0.20(Predicted)
- Flash Point:252.9 °C
- PSA:83.63000
- Density:1.416 g/cm3
- LogP:-1.29690
Oxiracetam(Cas 62613-82-5) Usage
|
Indications and Usage |
Oxiracetam is a potent derivative of the nootropic drug piracetam, developed by the Italian company ICF in 1974 and introduced to the market in 1987. As a Piracetam derivative, Oxiracetam excites the central nervous system, enhances brain metabolism, and notably improves memory retention. It is effective in treating senile memory and mental decline, as well as various brain dysfunctions, psychiatric syndromes, and mental disorders. Specifically, it demonstrates benefits in cases of senile dementia or memory disorders, neural dysfunction, memory disorder, and intelligence disorders resulting from brain damage. |
|
Mechanisms of Action |
Oxiracetam’s mechanisms of action are similar to those of Piracetam. It promotes the binding of phosphorylcholine and phosphoethanolamine, increases the ratio of ATP/ADP in the brain, and increases the binding of protein with nucleic acids in the brain, thus improving the memory and learning ability of patients with senile dementia and memory disorder. |
|
Pharmacokinetics |
After intake, most of the drug will not accumulate in the body and will be excreted through urine in its original form. It is well-absorbed and well-tolerated. |
|
Clinical Research |
Animal trials showed that in all types of behavioral experiments, Oxiracetam can improve memory retention and learning ability, reduce brain shocks’ damage on memory, and prevent the learning abilities of rats suffering from spontaneous hypertensive cerebrovascular injury from decreasing. It can also increase acetylcholine circulation in rats’ skin and hippocampus, thus increasing affinity for choline uptake. |
|
Chemical Properties |
Whie Solid |
|
Originator |
ISF (Italy) |
|
Uses |
Oxiracetam stands out for its high solubility in water, making it suitable for injection. Research indicates that it has higher pharmacological activity and more significant curative effects compared to Piracetam. Structurally analogous to piracetam, Oxiracetam enhances vigilance and memory. Studies have shown its superiority over piracetam, particularly in memory improvement. It has been extensively used for treating dementia, including Alzheimer's disease (AD), vascular dementia (VaD), and mixed forms. However, some studies, like the one by Green et al. (1992) on AD patients, failed to demonstrate a difference from placebo. In contrast, a trial by Maina et al. (1989) involving 289 patients with multi-infarct dementia (MID) found that oxiracetam reduced behavioral symptoms after 12 weeks of treatment. |
|
Brand name |
Neuromet |
|
Biochem/physiol Actions |
Nootropic agent, improves learning and memory, and prevents impairment of cognitive functions. Its mechanism of action in not yet known, but it is suggested to modulate glutamatergic and cholinergic neurotransmission. Protein kinase C may be involved. |
|
Purification Methods |
This nootropic (Alzheimer) drug is purified by recrystallisation from MeOH or aqueous Me2CO and dried in vacuo. [NMR, IR: Pifferi & Pinza Farmaco Ed Sci 32 602 1977, Banfi et al. Farmaco Ed Sci 39 16 1984, Gouilaev & Senning Brain Research Rev 19 180 1994.] |
InChI:InChI=1/C6H10N2O3/c7-5(10)3-8-2-4(9)1-6(8)11/h4,9H,1-3H2,(H2,7,10)
62613-82-5 Relevant articles
Interactions between oxiracetam, aniracetam and scopolamine on behavior and brain acetylcholine
Giacomo Spignoli, Giancarlo Pepeu
Pharmacology Biochemistry and Behavior Volume 27, Issue 3, July 1987, Pages 491-495
Oxiracetam (50 and 100 mg/kg IP) administered 30 min before scopolamine reduced the scopolamine-induced amnesic effect and decrease in acetylcholine level in the cortex and hippocampus, but not in the striatum. Lower and higher doses of oxiracetam were ineffective.
CNS Pharmacology and Clinical Therapeutic Effects of Oxiracetam
Itil, T. M.; Menon, G. N.*; Songar, A.*; Itil, K. Z.*
Clinical Neuropharmacology 9():p S70-72,
According to HZI Data Bank Classification Systems, oxiracetam is a vigilance-enhancing compound with some effects on spontaneous memory. The therapeutic effect of oxiracetam can be discriminated from placebo, and in comparison with piracetam, oxiracetam exhibits greater improvement in memory factor.
Preparation method 2 - (4 -hydroxy -2 - oxo -1 - pyrrolidinyl) acetamide (by machine translation)
-
Paragraph 0030; 0034; 0035; 0037; 0041; 0042, (2019/07/29)
The invention relates to the field, orga...
62613-82-5 Process route
-
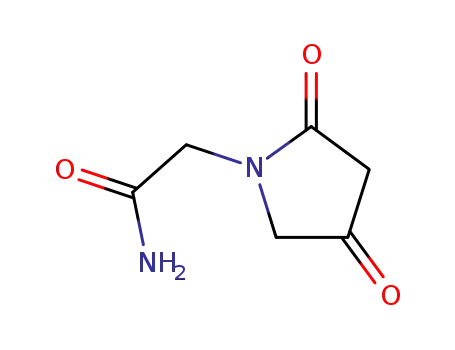
- 85614-54-6
2-(2,4-dioxopyrrolidine-1-yl)acetamide

-
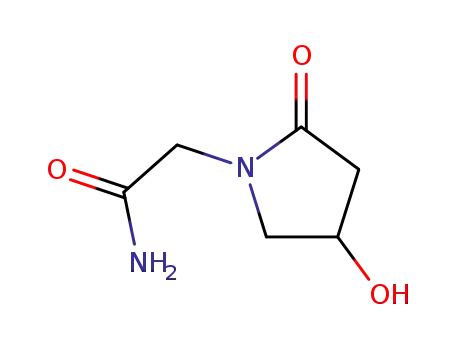
- 68252-28-8,68567-97-5,88929-35-5,62613-82-5
oxiracetam
| Conditions | Yield |
|---|---|
|
With sodium tetrahydroborate; In ethanol; dimethyl sulfoxide; at 0 - 35 ℃; for 4h; Reagent/catalyst;
|
78% |
|
With sodium tetrahydroborate; In dimethyl amine; at 15 ℃; for 1h; Yield given;
|
-
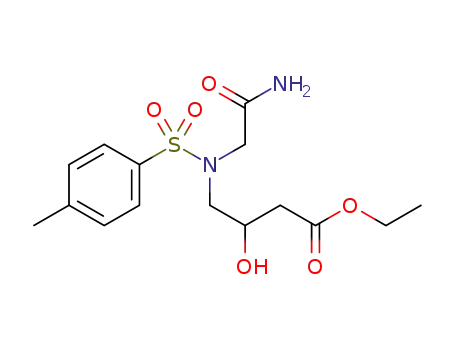
-
ethyl 4-(N-(2-acetamido)-N-p-toluenesulfonyl)-3-hydroxybutanoate

-

- 68252-28-8,68567-97-5,88929-35-5,62613-82-5
oxiracetam
| Conditions | Yield |
|---|---|
|
With methanesulfonic acid; trifluoroacetic acid; In tetrahydrofuran; at 25 ℃; for 4h; Solvent; Reagent/catalyst; Temperature;
|
87.6% |
62613-82-5 Upstream products
-
142274-09-7
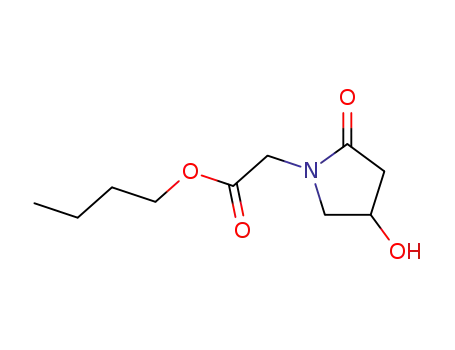
butyl (4-hydroxy-2-oxopyrrolidin-1-yl)acetate
-
142352-10-1
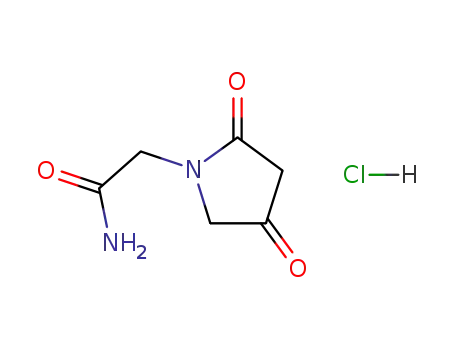
(2,4-dioxopyrrolidin-1-yl)acetamide hydrochloride
-
85614-54-6

2-(2,4-dioxopyrrolidine-1-yl)acetamide
-
85614-52-4
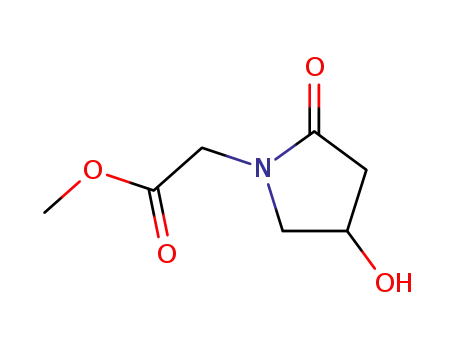
(4-Hydroxy-2-oxo-pyrrolidin-1-yl)-acetic acid methyl ester
62613-82-5 Downstream products
-
1392812-94-0
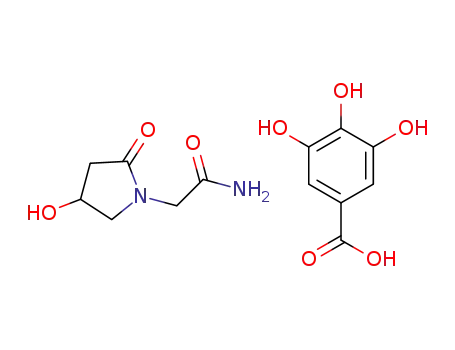
C6H10N2O3*C7H6O5
-
1392812-96-2
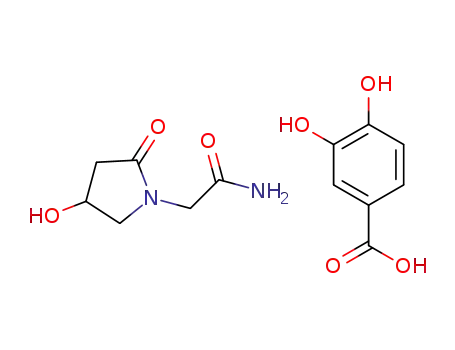
C6H10N2O3*C7H6O4
-
77191-37-8
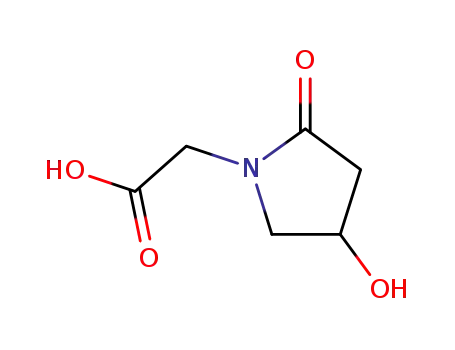
4-hydroxy-2-oxo-1-pyrrolidine acetic acid
-
62833-66-3
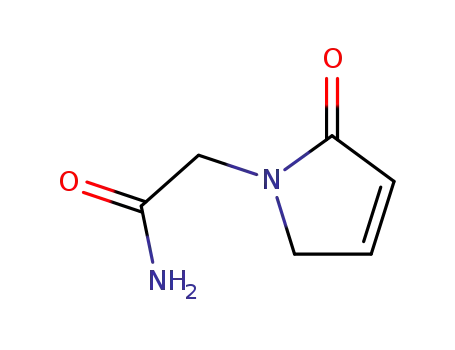
2,5-Dihydro-2-oxo-1H-pyrrol-1-acetamid
Relevant Products
-
Azithromycin dihydrate
CAS:117772-70-0
-
N-(2-Hydroxyethyl)-2-pyrrolidone
CAS:3445-11-2
-
1,4-Butanediol vinyl ether
CAS:17832-28-9