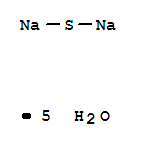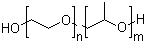
7757-82-6
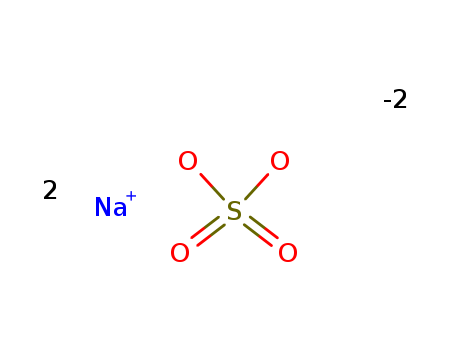
- Product Name:Sodium sulfate
- Molecular Formula:Na2SO4
- Purity:99%
- Molecular Weight:142.043
Product Details;
CasNo: 7757-82-6
Molecular Formula: Na2SO4
Appearance: white crystals or powder
Factory Supply High Purity Top Purity Sodium sulfate 7757-82-6 In Bulk Supply
- Molecular Formula:Na2SO4
- Molecular Weight:142.043
- Appearance/Colour:white crystals or powder
- Vapor Pressure:3.35E-05mmHg at 25°C
- Melting Point:884 °C
- Refractive Index:1.484
- Boiling Point:330 °C at 760 mmHg
- PSA:88.64000
- Density:2.68 g/mL at 25 °C(lit.)
- LogP:-0.25720
Sodium sulfate(Cas 7757-82-6) Usage
|
General Description |
Sodium sulfate, also known as sulfate of soda, is an inorganic compound with the chemical formula Na2SO4. It's a white crystalline solid that is odorless and highly soluble in water. This sodium salt of sulfuric acid is typically used in the manufacturing of detergents, glass, and paper, although it also has applications in textiles, the food industry, pulp and paper, and other various chemical reactions. Sodium sulfate is non-toxic but can cause redness or irritation when in contact with eyes and skin. While not considered highly hazardous, it should still be handled with care and stored properly to avoid accidental exposure or spillage. |
|
Who Evaluation |
Evaluation year: 1978 |
InChI:InChI=1/2Na.H2O4S/c;;1-5(2,3)4/h;;(H2,1,2,3,4)/q2*+1;/p-2
7757-82-6 Relevant articles
Investigation of 3,3′,5,5′-tetra-tert-butyl-4,4′-stilbenequinone-based catalyst in the reaction of liquid-phase oxidation of inorganic sulfides
Hoang, Hien Y.,Akhmadullin, Renat Maratovich,Akhmadullina, Farida Yunusovna,Zakirov, Rustem Kayumovich,Bui, Dinh Nhi,Akhmadullina, Alfiia Garipova,Gazizov, Almir Sabirovich
, p. 130 - 139 (2018)
In this paper, the intermediate and fina...
STUDIES ON THE CONCENTRATED STEFFEN FLUID OF BEET SUGAR INDUSTRY - 1. ON THE SALT SEPARATED FROM CONCENTRATED STEFFEN FLUID USING METHANOL AND SULFURIC ACID.
Ito,Abe,Sasaki,Wakabayashi,Misono
, p. 3920 - 3922 (1982)
The chemical desalting technique was app...
Lewis acid base reactions between boron trifluoride and complex oxoanions as a versatile access to fluorooxoanions: Synthesis of sodium (Trifluoroborato)sulfate
Pilz, Thomas,Jansen, Martin
, p. 733 - 736 (2012)
Na2SO4BF3, synthesized in a closed vesse...
FeCl2-Na2SO3-H2O system as a basis for recovery of iron(II) sulfite from solution
Motov,Vasekha
, p. 1674 - 1677 (2009)
The FeCl2-Na2SO3-H2O system was studied ...
Evolved gas analyses on a mixed valence copper(I,II) complex salt with thiosulfate and ammonia by in situ TG-EGA-FTIR and TG/DTA-EGA-MS
Madarasz, Janos
, p. 111 - 116 (2009)
Thermal decomposition of a mixed valence...
Structural, transport, and electrochemical investigation of novel AMSO 4F (A = Na, Li; M = Fe, Co, Ni, Mn) metal fluorosulphates prepared using low temperature synthesis routes
Barpanda, Prabeer,Chotard, Jean-Nol,Recham, Nadir,Delacourt, Charles,Ati, Mohamed,Dupont, Loic,Armand, Michel,Tarascon, Jean-Marie
, p. 7401 - 7413 (2010)
We have recently reported a promising 3....
Studies on synthetic galloalunites AGa3(SO4) 2(OH)6: Synthesis, thermal analysis, and X-ray characterization
Rudolph, Wolfram W.,Schmidt, Peer
, p. 112 - 120 (2011)
Stoichiometric end member galloalunites ...
LXXXVI. - The hydrogen sulphates of the alkali metals and ammonium
Dunnicliff, Horace Barratt
, p. 731 - 738 (1923)
-
Spectral, thermal, and photochemical studies on certain first, second, and third generation cephalosporin antibiotics and their Cd(II) complexes
Osman, Ahmed H.,El-Maali, Nagwa Abo,Aly, Aref A. M.,Al-Hazmi, Gamil A. A.
, p. 763 - 781 (2002)
The reactions of six cephalosporin antib...
Thermal characteristics of novel NaH2PO4/NaHSO 4 flame retardant system for polyurethane foams
Kulesza,Pielichowski,Kowalski
, p. 475 - 478 (2006)
Thermal behaviour of NaH2PO4/NaHSO4 flam...
Study of thermal preparation of iron (III) pigments by means of thermal analysis methods
Solc,Trojan,Brandova,Kuchler
, p. 463 - 469 (1988)
The decomposition of hydronium jarosite ...
Water-dissolvable sodium sulfate nanowires as a versatile template for the fabrication of polyelectrolyte- and metal-based nanotubes
Pu, Ying-Chih,Hwu, Jih Ru,Su, Wu-Chou,Shieh, Dar-Bin,Tzeng, Yonhua,Yeh, Chen-Sheng
, p. 11606 - 11611 (2006)
This study presents the synthesis of wat...
On the Possibility of Replacing Standard Chromium-Plating Electrolytes with Sulfate-Oxalate Solutions of Cr(III)
Edigaryan,Polukarov
, p. 323 - 324 (2003)
A method for preparing sulfate-oxalate c...
Cation-tuned synthesis of the A2SO4·SbF3 (A = Na+, NH4+, K+, Rb+) family with nonlinear optical properties
He, Fangfang,Wang, Lei,Hu, Cuifang,Zhou, Jing,Li, Qian,Huang, Ling,Gao, Daojiang,Bi, Jian,Wang, Xin,Zou, Guohong
, p. 17486 - 17492 (2018)
Four antimony fluoride sulfates named A2...
Liquid-phase oxidation of hydrogen sulfide in centrifugal bubbling apparatus
Ryazantsev,Malikov,Batoeva,Faddeenkova
, p. 1544 - 1548 (2007)
Liquid-phase oxidation of H2S in centrif...
Synthesis, characterization, and luminescence studies of rare-earth-activated NaMgF3
Singh, Vartika S.,Belsare, Pankaj D.,Moharil, Sanjiv V.
, p. 89 - 96 (2021/11/09)
NaMgF3-based phosphors have been describ...
PROCESS
-
, (2021/09/26)
In a process for treating wastewater fro...
OPEN-FLASK HYDROBORATION AND THE USE THEREOF
-
Paragraph 0042, (2018/03/25)
The present disclosure generally relates...
7757-82-6 Process route
-

- 10097-32-2
bromine

-
-
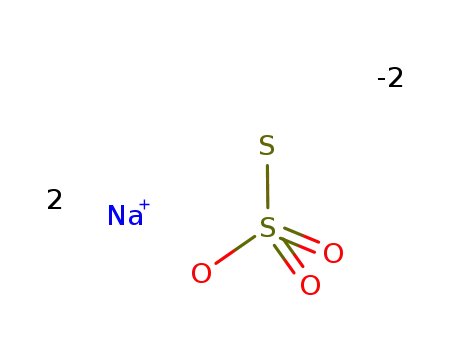
sodium thiosulfate

-

- 7664-93-9
sulfuric acid

-
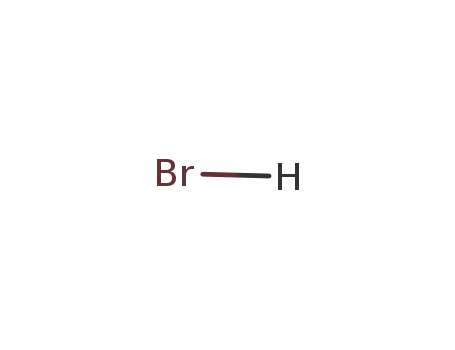
- 10035-10-6,12258-64-9
hydrogen bromide

-
- 7757-82-6
sodium sulfate
| Conditions | Yield |
|---|---|
|
With water; exclusion of oxygen, excess of thiosulfate, too;
|
|
|
With H2O; exclusion of oxygen, excess of thiosulfate, too;
|
-
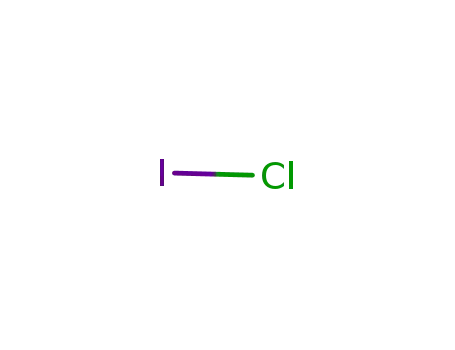
- 7790-99-0
Iodine monochloride

-
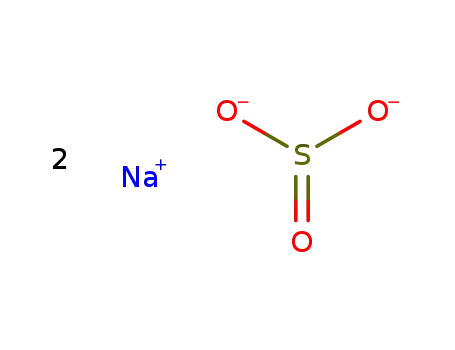
- 7757-83-7
sodium sulfite

-
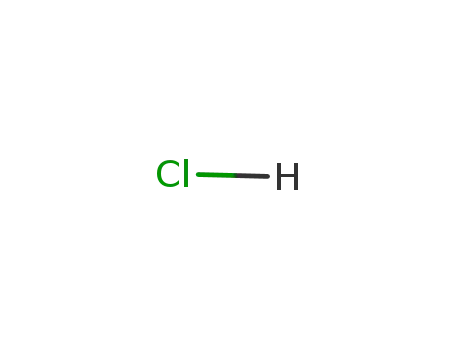
- 7647-01-0,15364-23-5
hydrogenchloride

-
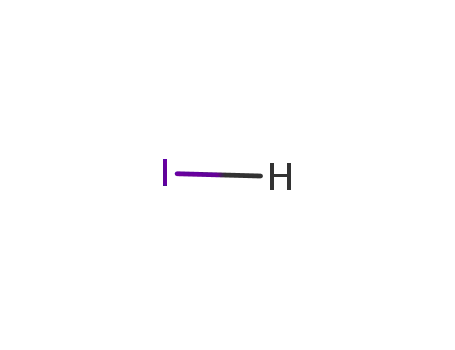
- 10034-85-2
hydrogen iodide

-

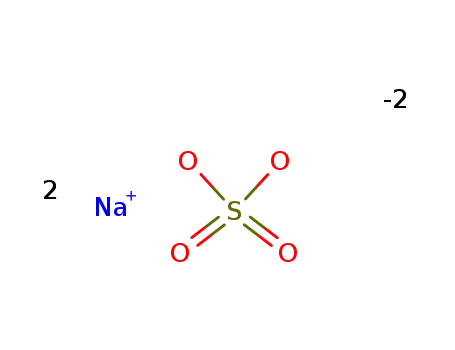
- 7757-82-6
sodium sulfate
| Conditions | Yield |
|---|---|
|
|
7757-82-6 Upstream products
-
7757-83-7

sodium sulfite
-
20244-21-7
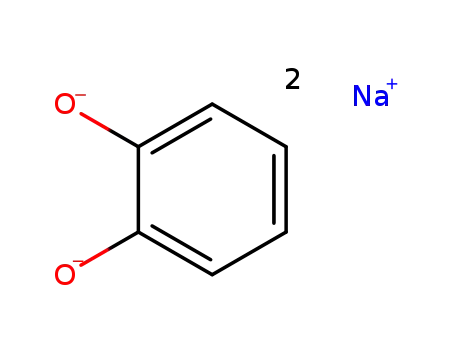
sodium catecholate
-
139-02-6
sodium phenoxide
-
7664-46-2
disodium salt of hydroquinone
7757-82-6 Downstream products
-
353240-66-1
1-amino-3-(4-bromo-2-fluoro-5-nitrophenyl)-6-tri-fluoromethyl-2,4-(1H,3H)-pyrimidinedione
-
364592-74-5
N-[5-(3-amino-2,6-dioxo-4-trifluoromethyl-3,6-dihydro-1(2H)-pyrimidinyl)-2-cyano-4-fluoro-phenyl]-N-benzoyl-1-ethanesulphonamide
-
99168-05-5
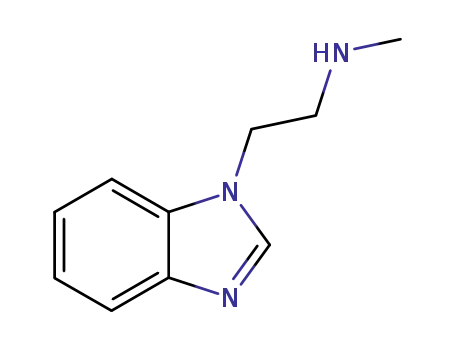
1-(2-methylamino-ethyl)-1H-benzo[d]imidazole
-
259195-70-5
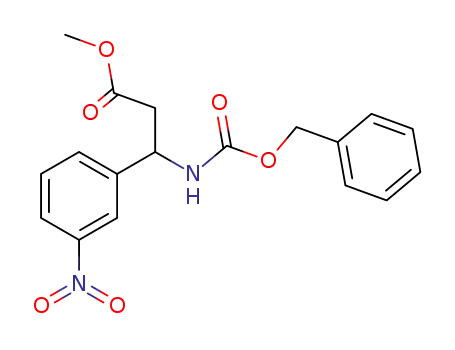
methyl 3-benzyloxycarbonylamino-3-(3-nitrophenyl)propionate
Relevant Products
-
SODIUM SULFIDE PENTAHYDRATE
CAS:1313-83-3
-
Polyethylene-polypropylene glycol
CAS:9003-11-6
-
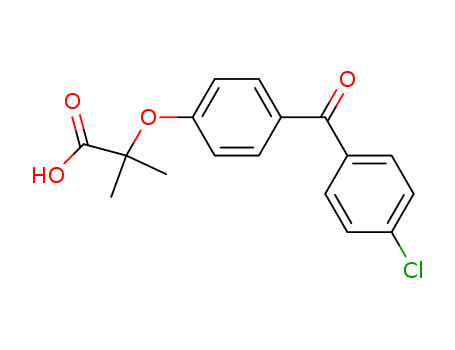
Fenofibric acid
CAS:42017-89-0