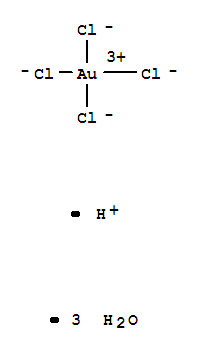
42017-89-0
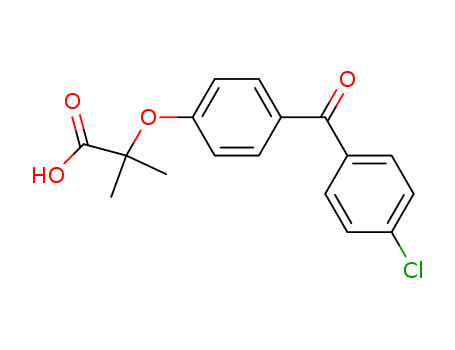
- Product Name:Fenofibric acid
- Molecular Formula:C17H15ClO4
- Purity:99%
- Molecular Weight:318.757
Product Details;
CasNo: 42017-89-0
Molecular Formula: C17H15ClO4
Appearance: white to off-white solid
Buy Reliable Quality Wholesale Fenofibric acid 42017-89-0 Cheapest Price
- Molecular Formula:C17H15ClO4
- Molecular Weight:318.757
- Appearance/Colour:white to off-white solid
- Vapor Pressure:2.82E-10mmHg at 25°C
- Melting Point:177-179 °C
- Boiling Point:486.457 °C at 760 mmHg
- PKA:3.09±0.10(Predicted)
- Flash Point:248.001 °C
- PSA:63.60000
- Density:1.287 g/cm3
- LogP:3.81300
Fenofibric acid(Cas 42017-89-0) Usage
|
Description |
Fenofibric acid is a medication that effectively lowers triglyceride levels and increases good cholesterol levels in the blood. It is the active metabolite of fenofibrate. The compound works by enhancing the transcription of the ATP-binding cassette transporter A1 gene in a liver X receptor-dependent manner, thereby increasing Apolipoprotein A-I-mediated High-Density Lipoprotein (HDL) biogenesis. |
|
Uses |
This fibrate is prescribed to address severe hypertriglyceridemia, primary hypercholesterolemia, or mixed dyslipidemia. By reducing cholesterol and triglycerides, fenofibric acid plays a vital role in managing lipid levels and promoting cardiovascular health. |
|
Definition |
ChEBI: A monocarboxylic acid that is 2-methylpropanoic acid substituted by a 4-(4-chlorobenzoyl)phenoxy group at position 2. It is a metabolite of the drug fenofibrate. |
|
Consumer Uses |
ECHA has no public registered data indicating whether or in which chemical products the substance might be used. ECHA has no public registered data on the routes by which this substance is most likely to be released to the environment. |
InChI:InChI=1/C17H15ClO4/c1-17(2,16(20)21)22-14-9-5-12(6-10-14)15(19)11-3-7-13(18)8-4-11/h3-10H,1-2H3,(H,20,21)/p-1
42017-89-0 Relevant articles
Interesting morphological behavior of organic salt choline fenofibrate: Effect of supersaturation and polymeric impurity
Bordawekar, Shailendra,Kuvadia, Zubin,Dandekar, Preshit,Mukherjee, Samrat,Doherty, Michael
, p. 3800 - 3812 (2014)
Crystal habit of drug molecules can have...
Management of dyslipidemias with fibrates, alone and in combination with statins: role of delayed-release fenofibric acid
Elisavet Moutzouri,Anastazia Kei,Moses S Elisaf &Haralampos J Milionis
, Vascular Health and Risk Management Volume 6, 2010 - Issue
Fenofibrate is the most commonly used fibric acid derivative, exerts beneficial effects in several lipid and nonlipid parameters, and is considered the most suitable fibrate to combine with a statin. However, in clinical practice this combination raises concerns about safety. ABT-335 (fenofibric acid, Trilipix®) is the newest formulation designed to overcome the drawbacks of older fibrates, particularly in terms of pharmacokinetic properties.
Targeting lipid metabolism in multiple myeloma cells: Rational development of a synergistic strategy with proteasome inhibitors
Xu, Gaojie,Huang, Sheng,Peng, Jian,Gao, Xiaofang,Li, Minhui,Yu, Sisi,Liu, Zuofeng,Qie, Pengfan,Wang, Yu,Yu, Siqi,Liu, Siyuan,Wen, Hu,Su, Lijuan,Li, Ping,Guang, Bin,Dong, Renhan,Liu, Jin,Yang, Tai
, p. 4741 - 4757 (2021)
Background and Purpose: Aberrant lipid m...
42017-89-0 Process route
-
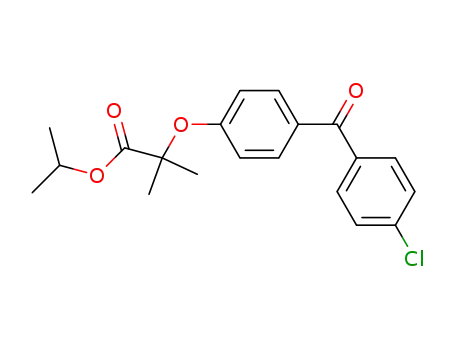
- 49562-28-9
fenofibrate

-
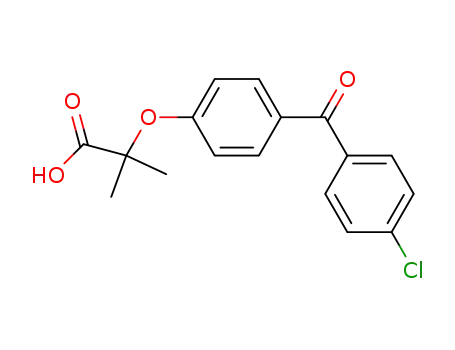
- 42017-89-0
fenofibric acid
| Conditions | Yield |
|---|---|
|
With water; triethylamine; lithium bromide; In acetonitrile; for 7h; Heating;
|
99% |
|
With iodine; aluminium; In acetonitrile; at 80 ℃; for 18h;
|
97% |
|
With sodium hydroxide; In methanol; at 40 ℃; for 10h;
|
93.1% |
|
With water; sodium hydroxide; In methanol; at 40 ℃; for 10h;
|
93.1% |
|
With sodium hydroxide; In methanol; at 69.85 ℃; for 4h;
|
85% |
|
fenofibrate; With lithium hydroxide monohydrate; water; In tetrahydrofuran; Reflux;
With hydrogenchloride; In tetrahydrofuran; water;
|
15% |
|
With lithium hydroxide monohydrate; water; In tetrahydrofuran; Reflux;
|
15% |
|
With sodium hydroxide; In methanol; water; for 5h; Heating;
|
|
|
With sodium hydroxide; In water; acetonitrile; at 90 ℃; for 2.5h;
|
|
|
With sodium hydroxide; In methanol; at 40 ℃; for 0.75h; Further Variations:; Reagents; Solvents; Temperatures; pH-values; Kinetics;
|
|
|
fenofibrate; With sodium hydroxide; In isopropyl alcohol; at 82 ℃; for 2h; Reflux;
With hydrogenchloride;
|
|
|
With carboxylesterase;
|
|
|
With sodium hydroxide; for 4h; Reflux;
|
|
|
With ethanol; sodium hydroxide; at 60 - 80 ℃; for 1h;
|
60.1 g |
-
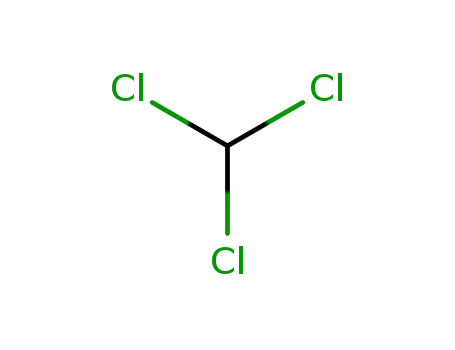
- 67-66-3,8013-54-5
chloroform

-
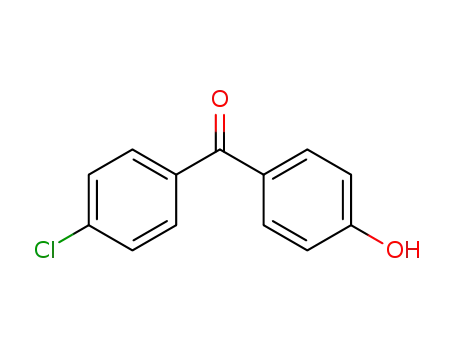
- 42019-78-3
4-chloro-4'-hydroxybenzophenone

-
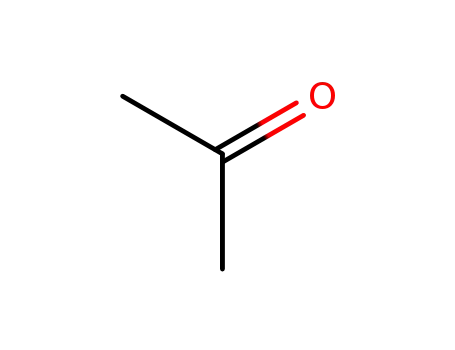
- 67-64-1
acetone

-

- 42017-89-0
fenofibric acid
| Conditions | Yield |
|---|---|
|
4-chloro-4'-hydroxybenzophenone; acetone; With sodium hydroxide; for 2h; Heating / reflux;
chloroform; In acetone; for 8h; Heating / reflux;
|
73% |
|
With sodium hydroxide;
|
|
|
With sodium hydroxide;
|
42017-89-0 Upstream products
-
67-66-3

chloroform
-
42019-78-3

4-chloro-4'-hydroxybenzophenone
-
67-64-1

acetone
-
49562-28-9

fenofibrate
42017-89-0 Downstream products
-
61002-01-5
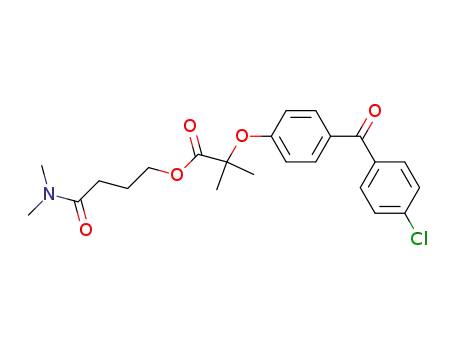
2-[4-(4-Chloro-benzoyl)-phenoxy]-2-methyl-propionic acid 3-dimethylcarbamoyl-propyl ester
-
49562-28-9

fenofibrate
-
65178-90-7
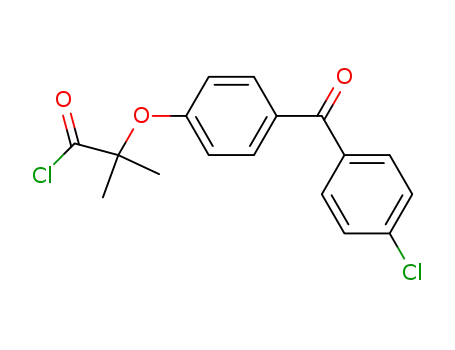
2-[4-(4-chlorobenzoyl)-phenoxy]-2-methylpropionyl chloride
-
154356-96-4
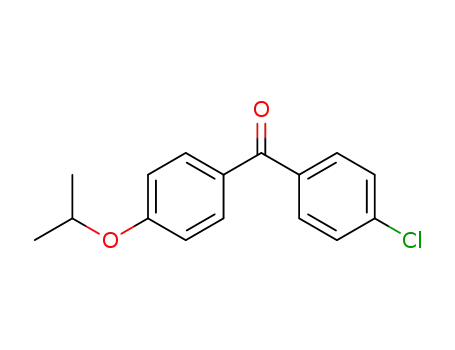
(4-chlorophenyl)(4-isopropoxyphenyl)methanone
Relevant Products
-
HYDROGEN TETRACHLOROAURATE(III)
CAS:16961-25-4
-
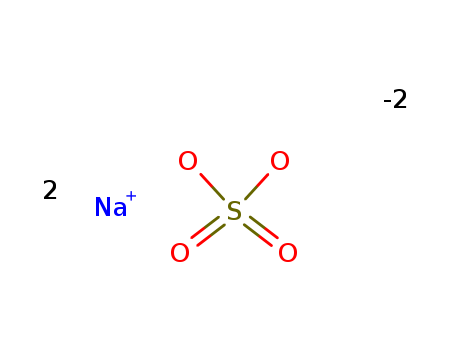
Sodium sulfate
CAS:7757-82-6
-
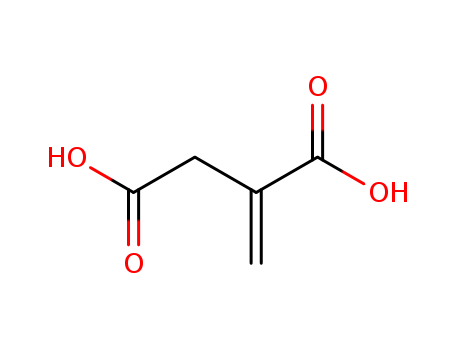
Itaconic acid
CAS:97-65-4