10592-13-9
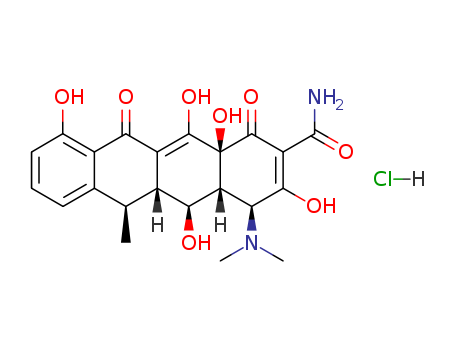
- Product Name:Doxycycline hydrochloride
- Molecular Formula:C22H24N2O8.HCl
- Purity:99%
- Molecular Weight:480.902
Product Details;
CasNo: 10592-13-9
Molecular Formula: C22H24N2O8.HCl
Appearance: White crystalline powder
Buy Quality High Purity Doxycycline hydrochloride 10592-13-9 Low Price
- Molecular Formula:C22H24N2O8*ClH
- Molecular Weight:480.902
- Appearance/Colour:White crystalline powder
- Melting Point:195-201℃
- Boiling Point:762.6 °C at 760 mmHg
- Flash Point:415 °C
- PSA:181.62000
- Density:1.63 g/cm3
- LogP:1.15470
Doxycycline hydrochloride(Cas 10592-13-9) Usage
|
Overview |
Doxycycline hydrochloride is a tetracycline antibiotic widely used in both veterinary and human medicine due to its broad spectrum and safety margin. Discovered in 1967, it has become one of the most commonly prescribed and cost-effective broad-spectrum antibiotics globally. In Australia alone, over 800,000 annual prescriptions were recorded between 2002-2005. |
|
Origin and structure |
The development of tetracycline antibiotics was the result of a systematic screening of soil specimens collected from many parts of the world for antibiotic-producing organisms. A number of tetracyclines exist, all very much alike in structure and function. Tetracycline and doxycycline are semi-synthetically produced from a species of Streptomyces and differ only by the position of a single hydroxy moiety on carbon#5.[5] ?? Figure 1 the chemical structure of doxycycline and tetracycline. ; |
|
Mode of action |
The site of action of tetracycline and doxycycline is the bacterial ribosome. The drugs bind primarily to 30 S ribosomes and block access of bacterial aminoacyl tRNA to the acceptor sites on mRNA, preventing the addition of amino acids to the growing peptide chain. Resistance is mediated by a plasmid that prevents the intracellular accumulation of tetracyclines. These drugs are primarily bacteriostatic, preventing the organism from reproducing but not killing it outright. Thus, an intact immune system is necessary for eradication of the infection[5, 21] |
|
Adverse reactions and precaution |
The most common adverse events described for doxycycline include the oesophageal erosion and photosensitivity (reported as a 10% risk), there were only 130 described between 1966 and 2003 and the denominator is unknown,[4] but it is likely that the number of prescriptions for doxycycline during this period was probably 100’s of millions. There are, however, wider ranges of side effects, which may present with the use of doxycycline, many of which may be reduced by some common sense measures. It should be noted that side effects are not limited to medical practice and may be seen in dental practice as well.[24] Some of the more unusual side effects include photo-onycholysis, various skin eruptions, Stevens-Johnson syndrome, Jarisch-Herxheimer reaction, and benign intracranial hypertension, as well as a potential risk of hepatotoxicity, which is thought to be low compared to other tetracyclines.[2] Doxycycline should not be used by pregnant or lactating women or by children younger than 8 years because of its deleterious effects on bone and tooth development.[2] Also, it should not be used by persons with known allergy or sensitivity to the drug.[4, 22] Some more tips are summarized below: Take with a meal Do not lie down for at least 30 minutes to 1 hour after taking the drug to prevent its reflux into the esophagus resulting in esophagitis Swallowed with a full glass of water to ensure that it does not get stuck in the esophagus, leading to esophageal ulceration Women using doxycycline should be recommended to obtain an over-the counter antifungal drug or prescribed an antifungal drug to treat vaginal candidiasis, if they become symptomatic Avoid peak (midday) sun exposure Use a sunscreen preparation that contains an ultraviolet A (UVA) blocker since the phototoxicity associated with doxycycline is UVA induced. |
|
Chemical Properties |
Yellow Crystalline powder |
|
Uses |
Respiratory and Genitourinary Infections: Doxycycline is employed in the treatment of respiratory tract infections and infections caused by various bacteria, including staphylococci. Chronic Conditions: It is used for common chronic conditions such as acne and rosacea. Unusual Infectious Diseases: Known for its efficacy against "atypical bacteria," doxycycline is considered a "wonder drug" or "secret weapon" in treating unusual infectious diseases. Dental Applications: Doxycycline finds applications in various dental conditions. Bioterrorism Agents: Following anthrax scares in 2000-2001, there was a 30% increase in prescriptions. It could potentially be used against other bioterrorist agents like tularemia and plague. Parasitic Infections: Future applications may involve treating parasitic infections such as lymphatic filariasis. Malaria: Doxycycline is widely used as an antimalarial prophylaxis, particularly in areas with chloroquine-resistant strains. Recent trials show protective efficacy ranging from 92% to 100%. |
|
Definition |
ChEBI: The hydrochloride salt of doxycycline. |
InChI:InChI=1/C22H24N2O8.ClH/c1-7-8-5-4-6-9(25)11(8)16(26)12-10(7)17(27)14-15(24(2)3)18(28)13(21(23)31)20(30)22(14,32)19(12)29;/h4-7,10,14-15,17,25,27-29,32H,1-3H3,(H2,23,31);1H/t7-,10+,14+,15-,17-,22-;/m0./s1
10592-13-9 Relevant articles
PENTACYCLINE DERIVATIVES FOR THE TREATMENT OF INFECTIONS
-
Paragraph 0058; 0218-0219; 0251; 0258-0259, (2020/07/02)
The tetracycline class of antibiotics ha...
A robust platform for the synthesis of new tetracycline antibiotics
Sun, Cuixiang,Wang, Qiu,Brubaker, Jason D.,Wright, Peter M.,Lerner, Christian D.,Noson, Kevin,Charest, Mark,Siegel, Dionicio R.,Wang, Yi-Ming,Myers, Andrew G.
supporting information; experimental part, p. 17913 - 17927 (2009/07/18)
Tetracyclines and tetracycline analogues...
The Effect of Doxycycline Hyclate, Chlorhexidine Gluconate, and Minocycline Hydrochloride on Osteoblastic Proliferation and Differentiation In Vitro
Salah M. Almazin, Rosemary Dziak, Sebastiano Andreana, Sebastian G. Ciancio
Journal of Periodontology, Volume80, Issue6 June 2009 Pages 999-1005
The purpose of this study was to determine the effect of the active substance of three types of local delivery systems, doxycycline hyclate 10% (DOXY), chlorhexidine gluconate, 2.5 mg (CHX), and minocycline hydrochloride, 1 mg (MINO), on osteoblastic cell proliferation and differentiation.
10592-13-9 Process route
-
-
C37H34N2O10

-
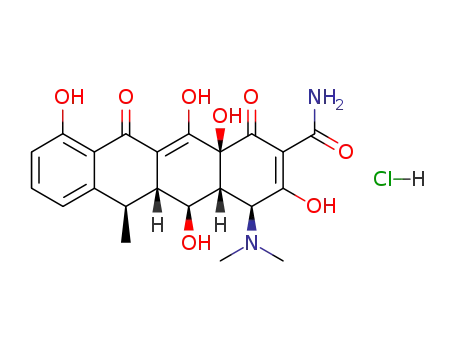
- 10592-13-9,41411-66-9,564-25-0,61281-03-6
doxycycline hydrochloride
| Conditions | Yield |
|---|---|
|
C37H34N2O10; With hydrogen; palladium; In tetrahydrofuran; methanol; at 23 ℃; for 2h; under 760.051 Torr;
With hydrogenchloride; In methanol; water; HPLC;
|
90% |
|
C37H34N2O10; With hydrogen; palladium; In tetrahydrofuran; methanol; at 23 ℃; for 2h; under 760.051 Torr;
With hydrogenchloride; In methanol; water; acetonitrile; Inert atmosphere;
|
90% |
-
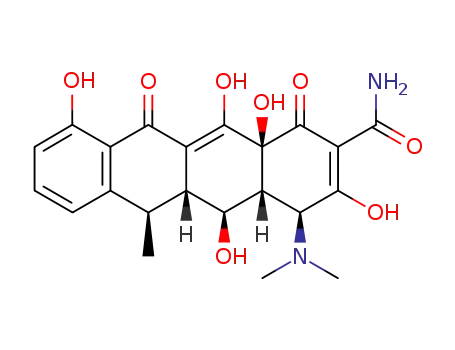
- 564-25-0,10597-92-9,7164-70-7,7264-10-0
doxycycline

-

- 10592-13-9,41411-66-9,564-25-0,61281-03-6
doxycycline hydrochloride
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; In methanol; water;
|
90% |
10592-13-9 Upstream products
-
564-25-0

doxycycline
-
32359-20-9
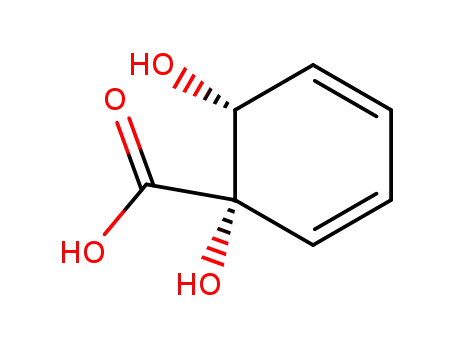
(1S,2R)-1,2-dihydroxycyclohexa-3,5-diene-1-carboxylic acid
-
367954-24-3
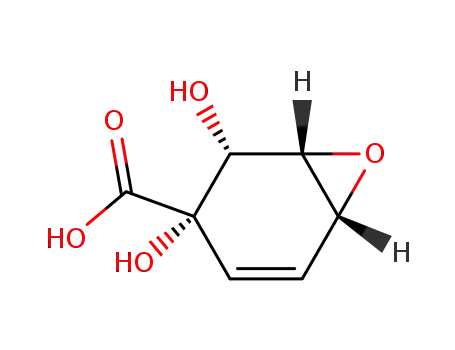
(1S,2R,3S,6R)-2,3-Dihydroxy-7-oxa-bicyclo[4.1.0]hept-4-ene-3-carboxylic acid
-
367954-37-8
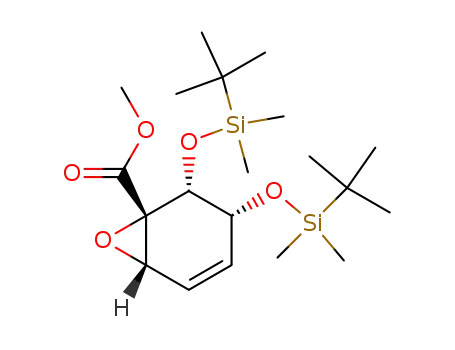
(1S,2R,3R,6S)-2,3-Bis-(tert-butyl-dimethyl-silanyloxy)-7-oxa-bicyclo[4.1.0]hept-4-ene-1-carboxylic acid methyl ester
10592-13-9 Downstream products
-
564-25-0

doxycycline
-
141-82-2
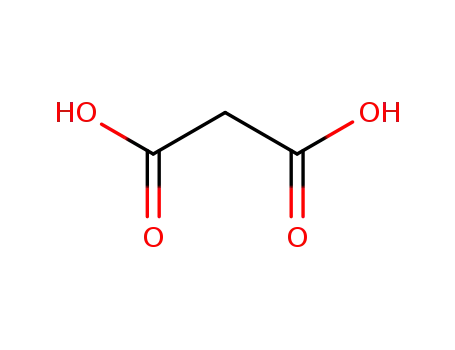
malonic acid
-
86271-83-2
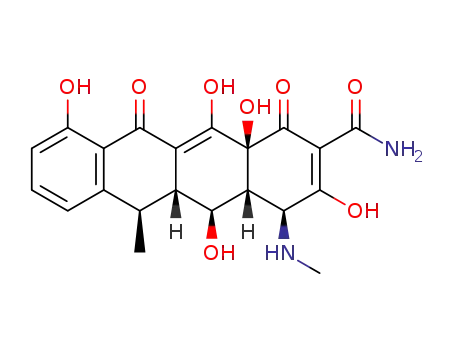
(4S,4aR,5S,5aR,6R,12aS)-3,5,10,12,12a-pentahydroxy-6-methyl-4-(methylamino)-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide
Relevant Products
-
Ciprofloxacin HCl
CAS:93107-08-5
-
MITRAGYNINE
CAS:6202-22-8
-
ALTRENOGEST
CAS:55720-26-8