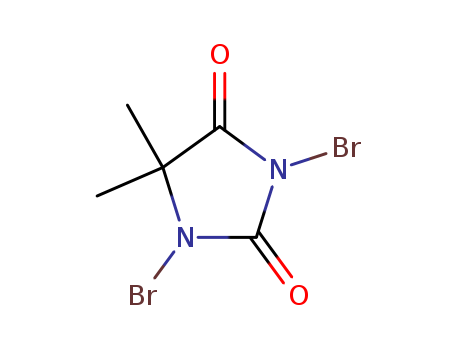
77-48-5
- Product Name:1,3-Dibromo-5,5-dimethylhydantoin
- Molecular Formula:C5H6Br2N2O2
- Purity:99%
- Molecular Weight:285.923
Product Details;
CasNo: 77-48-5
Molecular Formula: C5H6Br2N2O2
Appearance: white powder
Quality Manufacturer Supply Hot Sale 1,3-Dibromo-5,5-dimethylhydantoin 77-48-5 Best Price
- Molecular Formula:C5H6Br2N2O2
- Molecular Weight:285.923
- Appearance/Colour:white powder
- Vapor Pressure:0.022mmHg at 25°C
- Melting Point:197-199 °C (dec.)(lit.)
- Refractive Index:1.621
- Boiling Point:250.2 °C at 760 mmHg
- PKA:-3.44±0.40(Predicted)
- Flash Point:105.1 °C
- PSA:40.62000
- Density:2.183 g/cm3
- LogP:1.52480
1,3-Dibromo-5,5-dimethylhydantoin(Cas 77-48-5) Usage
|
Industrial brominating agent and disinfectant |
1,3-Dibromo-5,5-dimethylhydantoin (DBDMH), commonly known as DBDMH, serves as a versatile industrial brominating agent and an antiseptic disinfectant with a broad range of applications. Its advantages, including high active bromine content, excellent storage stability, and cost-effectiveness, make it widely utilized in the chemical, pharmaceutical, and agricultural industries. |
|
Physical and Chemical Properties |
1, 3-Dibromo-5, 5-dimethylhydantoin has the English abbreviation be DBDMH with pure product being white solid and the mp being 196~198 ℃. The industrial products appear as a pale yellow solid with a mp of 194~197 ℃; it is soluble in chloroform, ethanol, acetone and other organic solvents, slightly soluble in water with 1 L water being able to dissolve 2.2 g of DBDMH at 20℃ with the pH of 0.1% aqueous solution being 2.6; It is prone to be subject to decomposition in strong acid or alkali; it is stable when dry and is easy to absorb moisture with partially hydrolysis after moisture absorption; it has a slight pungent odor with the active bromine content being 54% to 55%. |
|
Sterilization Mechanism |
DBDMH, upon hydrolysis in water, can mainly form hypobromous acid and can release bromine in the form of hypobromous acid. DBDMH, due to able to release bromine in a high reaction rate, can continuously release Br-ions in the water, further playing a bactericidal effect. However, other kinds of halogenated hydantoin, such as 1, 3-Dibromo-5, 5-dimethylhydantoin or bromochlorohydantoin, release the chlorine in a very slow rate, leading to that the time for reaching the peak of sterilization in the water is lagging behind, therefore, DBDMH usually has a higher efficiency than other kind of halogenated hydantoin at relatively rapid bactericidal conditions. |
|
Synthesis of 1,3-Dibromo-5,5-dimethylhydantoin |
Adding an appropriate amount of water to dimethyl hydantoin, control the temperature at about 30 ℃ for stirring of 30min until all the dimethyl hydantoin is dissolved. Add drop wise of appropriate amount of bromine within a certain period of time and then have reaction for several hours. After the completion of the reaction, cool the solution, crystallize using ethanol, filter, and dry with the light-yellow solid being finished product of DBDMH. The optimal condition for experimentally determination and synthesis of DBDMH is: the optimum molar ratio of DMH to bromine is 1: 2.0; the solvent amount is 80 mL (water) /0.1mol (Hein); the reaction temperature should be about 30 ℃; Reaction time is around 5min with the yield of 1,3-dibromo-5,5-dimethylhydantoin being up to 80%. |
|
Uses |
In chemical and pharmaceutical applications, DBDMH is employed for the bromination of allyl and benzyl compounds, aromatic ring bromination, addition reactions involving bromine in double bonds, hydrogen bromide addition, and selective oxidation of secondary alcohols. Its efficacy extends to serving as a potent disinfectant, effectively eliminating fungi, bacteria, viruses, and adverse algae in various settings. Applications range from aquaculture disease prevention and treatment in fish, shrimp, frogs, and turtles to disinfection in swimming pools, fruit preservation, and algae control in industrial recycled water and daily disinfection. Notably, during the SARS outbreak, experts recommended using a DBDMH solution with an effective bromine concentration of 500-1000mg/L for spray, mopping, and cleaning, achieving disinfection within 10-15 minutes. DBDMH's significance is further emphasized by its combination with other halogenated hydantoins, such as BCDMH and DCDMH, in recent formulations. The effectiveness of these combinations is influenced by the ratio between effective chlorine and effective bromine. For instance, combining equal doses of BCDMH and DBDMH in a compound formulation at pH 7.1 exhibits higher bactericidal efficacy than using DBDMH or BCDMH alone. In agriculture, DBDMH finds utility in aquaculture, including pond disinfection, water disinfection, and disease treatment. Notably, its efficacy remains unaffected by water quality, salinity, pH, temperature, and organic compounds during usage. Additionally, there are successful cases of DBDMH being used in combination with stearic acid monoglyceride and Tween-80 as a high-efficiency, low-toxicity disinfectant for preventing mold and preserving oranges. This success opens up possibilities for using the same preservative for the storage and preservation of other fruits and vegetables. |
|
Chemical Properties |
It appears as white or light yellow crystalline powder. |
|
Category |
Pesticide |
|
Toxicity grading |
Highly toxic |
|
Acute toxicity |
Oral-rat LD50: 250 mg/kg. |
|
Flammability and hazard characteristics |
It is flammable with burning discharging spicy smoke of bromide and nitrogen oxides. |
|
Storage properties |
Warehouse: cold, ventilated and dry; store it separately from food raw materials. |
|
Extinguishing media |
Water, carbon dioxide, dry, sandy soil. |
|
Purification Methods |
Recrystallise it from H2O. Its solubility in CCl4 is 0.003 mol/L at 25o and 0.024 mol/L at 76.5o. [Beilstein 24 III/IV 1101.] |
|
Consumer Uses |
ECHA has no public registered data indicating whether or in which chemical products the substance might be used. ECHA has no public registered data on the routes by which this substance is most likely to be released to the environment. |
InChI:InChI=1/C5H6Br2N2O2/c1-5(2)3(10)8(6)4(11)9(5)7/h1-2H3
77-48-5 Relevant articles
Short inter-molecular N - Br...O=C contacts in 1,3-dibromo-5,5- dimethyl-imidazolidine-2,4-dione
Kruszynski, Rafal
, p. o389-o391 (2007)
In the title compound, C5H6Br2N2O2, all ...
Enantioselective 6-endo bromoaminocyclization of 2,4-dienyl N-tosylcarbamates catalyzed by a chiral phosphine oxide-Sc(OTf)3 complex. A dramatic additive effect
Huang, Hu,Pan, Hongjie,Cai, Yudong,Liu, Mao,Tian, Hua,Shi, Yian
, p. 3566 - 3570 (2015)
An effective enantioselective 6-endo bro...
1,3-Dibromo-5,5-Dimethylhydantoin [DBDMH] as an Efficient and Selective Agent for the Oxidation of Thiols to Disulfides in Solution or under Solvent-Free Conditions
Ardeshir Khazaei*, Mohammad Ali Zolfigol, Amin Rostami
Department of Chemistry, Faculty of Science, Bu-Ali Sina University, P.O. Box 65174-4119
A useful method for oxidation of various thiols to their corresponding disulfides with 1,3-dibromo-5,5-dimethylhydantoin (DBDMH) in very short reaction times and mild conditions under both solution and solvent-free conditions is described.
77-48-5 Process route
-
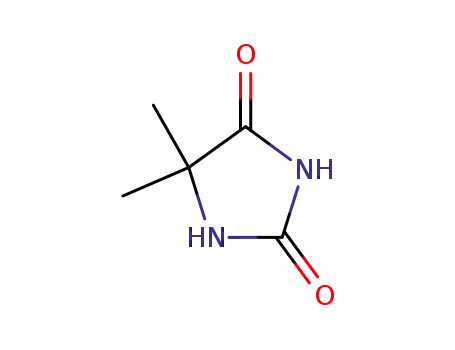
- 77-71-4
5,5-dimethyl-imidazolidine-2,4-dione

-
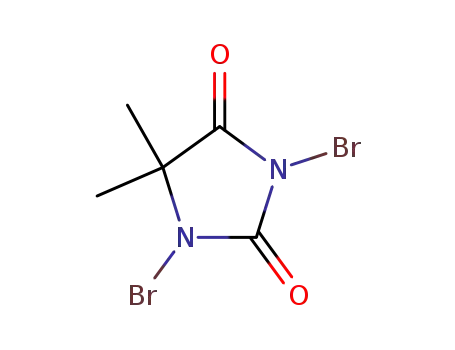
- 77-48-5
1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione
| Conditions | Yield |
|---|---|
|
With bromine;
|
99% |
|
With bromine;
|
99% |
|
With bromine;
|
99% |
|
With sodium hydroxide; bromine; In water; at 11.24 ℃; for 3h;
|
97.3% |
|
With sodium bromate; sulfuric acid; hydrogen bromide; In water; for 0.5h; Ambient temperature;
|
93% |
|
With sodium hydroxide; bromine; In water; at 25 - 94 ℃; pH=6.06 - 8.0; Conversion of starting material;
|
80% |
|
With bromine; sodium hydroxide; In water; at 30 ℃; for 0.583333h;
|
80% |
|
With bromine;
|
80% |
|
With bromine;
|
80% |
|
With bromine;
|
80% |
|
With sodium hydroxide; bromine;
|
|
|
5,5-dimethyl-imidazolidine-2,4-dione; With bromine; sodium hydroxide; In water; at -5 ℃; for 0.333333h;
With sodium 4-dodecylbenzenesulfonate; In water; at -5 ℃; for 0.333333h;
With sodium dihydrogenphosphate; In water; pH=4;
|
|
|
With bromine; sodium hydroxide; In water; at -5 - 35 ℃;
|
|
|
In water;
|
-
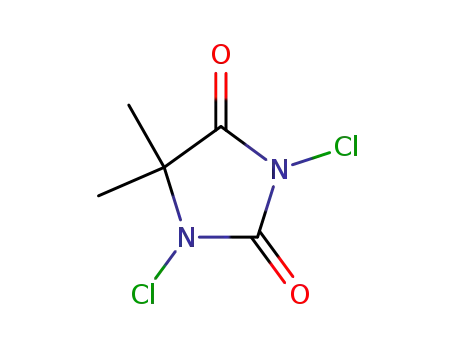
- 118-52-5
1,3-dichloro-5,5-dimethylhydantoin

-

- 77-71-4
5,5-dimethyl-imidazolidine-2,4-dione

-

- 77-48-5
1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione
| Conditions | Yield |
|---|---|
|
With sodium hydroxide; bromine; sodium carbonate;
|
77-48-5 Upstream products
-
118-52-5

1,3-dichloro-5,5-dimethylhydantoin
-
77-71-4

5,5-dimethyl-imidazolidine-2,4-dione
-
143-33-9
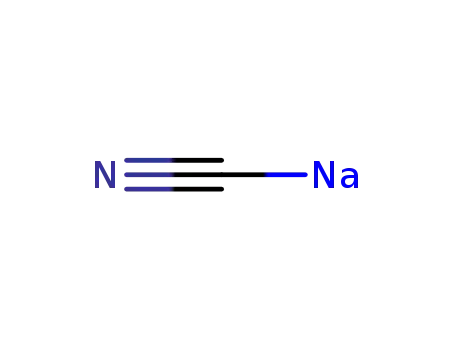
sodium cyanide
-
67-64-1
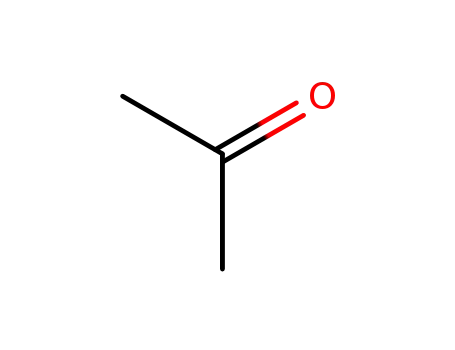
acetone
77-48-5 Downstream products
-
776-74-9
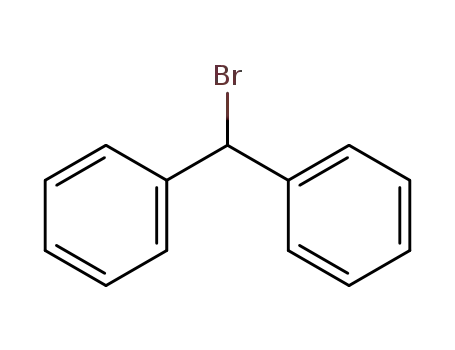
Bromodiphenylmethane
-
1940-57-4
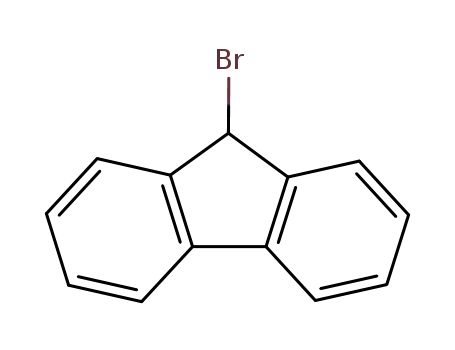
9H-fluoren-9-yl bromide
-
2859-78-1
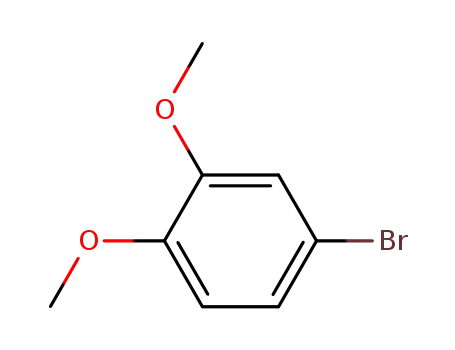
4-Bromoveratrole
-
3401-47-6
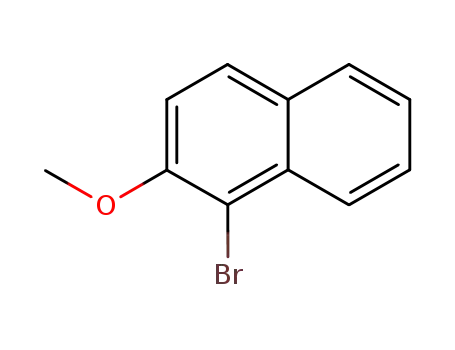
1-bromo-2-methoxynaphthalene
Relevant Products
-
Bromhexine hydrochloride
CAS:611-75-6
-
IGF-1 LR3
CAS:946870-92-4
-
MITRAGYNINE
CAS:6202-22-8