611-75-6
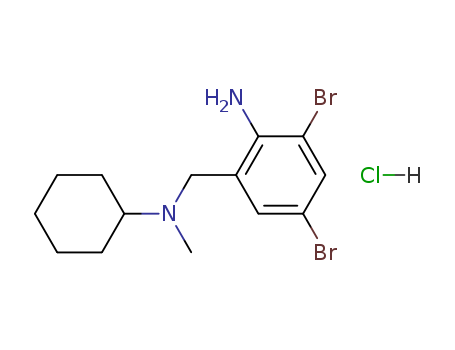
- Product Name:Bromhexine hydrochloride
- Molecular Formula:C14H20Br2N2.HCl
- Purity:99%
- Molecular Weight:412.595
Product Details;
CasNo: 611-75-6
Molecular Formula: C14H20Br2N2.HCl
Appearance: White Solid
Factory Supply High Purity Bromhexine hydrochloride 611-75-6 Efficient Transportation
- Molecular Formula:C14H20Br2N2.HCl
- Molecular Weight:412.595
- Appearance/Colour:White Solid
- Vapor Pressure:3.37E-08mmHg at 25°C
- Melting Point:240-244 °C
- Boiling Point:441.5 °C at 760 mmHg
- Flash Point:220.8 °C
- PSA:29.26000
- LogP:5.94150
Bromhexine hydrochloride(Cas 611-75-6) Usage
|
Description |
Bromhexine Hydrochloride is the hydrochloride salt form of bromhexine, characterized as a secretolytic agent with mucolytic activity. It operates by enhancing lysosomal activity, promoting the hydrolysis of acid mucopolysaccharide polymers in the respiratory tract. This process increases the production of serous mucus, leading to thinner phlegm and reduced mucus viscosity. The secretomotoric effect of bromhexine allows cilia to more efficiently transport phlegm out of the lungs. This mechanism is beneficial in treating respiratory disorders associated with abnormal viscid mucus, excessive mucus secretion, and impaired mucus transport. |
|
Chemical Properties |
White Solid |
|
Originator |
Bisolvon,Boehringer Ingelheim,Switz.,1963 |
|
Uses |
Respiratory Disorders: Bromhexine hydrochloride is employed for its therapeutic effects in respiratory conditions characterized by abnormal, thick mucus. It aids in clearing mucus from the respiratory tract. Pancreatic Juice Secretion: Historically, bromhexine hydrochloride has been utilized in the secretion of pancreatic juice with low viscosity. |
|
Definition |
ChEBI: A hydrochloride resulting from the reaction of equimolar amounts of bromhexine and hydrogen chloride. It is used as a mucolytic for the treatment of respiratory disorders associated with productive cough (i.e. a cough characterised by the production of spu um). |
|
Manufacturing Process |
In initial steps, 2-nitrobenzylbromide and cyclohexylmethylamine are reacted and that initial product reacted with hydrazine to give N-(2-aminobenzyl)-Nmethyl- cyclohexylamine. A solution of 29.3 g of bromine in 50 cc of glacial acetic acid was slowly added dropwise to a solution of 159 g of N-(2-aminobenzyl)-N-methylcyclohexylamine, accompanied by stirring. The glacial acetic acid was decanted from the precipitate formed during the addition of the bromine solution, and the precipitate was thereafter shaken with 200 cc of 2N sodium hydroxide and 600cc of chloroform until all of the solids went into solution. The chloroform phase was allowed to separate from the aqueous phase. The chloroform phase was decanted, evaporated to dryness and the residue was dissolved in absolute ether. The resulting solution was found to be a solution of N-(2-amino-3,5-dibromobenzyl)-N-methyl-cyclohexylamine in ethanol. Upon introducing hydrogen chloride into this solution, the hydrochloride of N-(2- amino-3,5-dibromobenzyl)-N-methyl-cyclohexylamine precipitated out. It had a melting point of 232°-235°C (decomposition). |
|
Therapeutic Function |
Expectorant; Mucolytic |
|
General Description |
Bromhexine hydrochloride is a bronchial mucolytic. |
InChI:InChI=1/C14H20Br2N2.ClH/c1-18(12-5-3-2-4-6-12)9-10-7-11(15)8-13(16)14(10)17;/h7-8,12H,2-6,9,17H2,1H3;1H
611-75-6 Relevant articles
Assay of bromhexine hydrochloride in pharmaceutical formulations by extraction spectrophotometry
SV Rao, IN Rao, T Reddy
Indian Journal of Chemical Technology (IJCT) IJCT Vol.12 [2005] IJCT Vol.12(2) [March 2005]
Beer’s law and the precision and accuracy of the methods are checked by the UV reference method. The results are reproducible with an accuracy of ± 1.0%. The methods are found to be suitable for the determination of bromhexine hydrochloride in the presence of the other ingredients that are usually present in dosage forms.
Respiratory Disorders: Bromhexine hydrochloride is employed for its therapeutic effects in respiratory conditions characterized by abnormal, thick mucus. It aids in clearing mucus from the respiratory tract. Pancreatic Juice Secretion: Historically, bromhexine hydrochloride has been utilized in the secretion of pancreatic juice with low viscosity.
Gubbi Sanjeev*, Jarag Ravindra
Research Journal of Pharmacy and Technology Year : 2009, Volume : 2, Issue : 2
The in vitro dissolution property of slightly water soluble Bromhexine hydrochloride (BXH) was improved by exploring the potential of Liquisolid system (LS). The in vitro release pattern of LS compacts and directly compressed tablets were studied using USP-II apparatus. Different LS compacts were prepared using a mathematical model to calculate the required quantities of powder and liquid ingredients to produce acceptably flowable and compressible admixture. Avicel PH 102, Aerosil 200 and Explotab were employed as carrier, coating material and disintegrant respectively for preparing LS compacts.
611-75-6 Process route
-
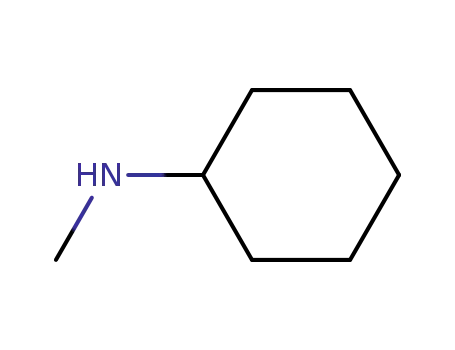
- 100-60-7
N-methylcyclohexylamine

-
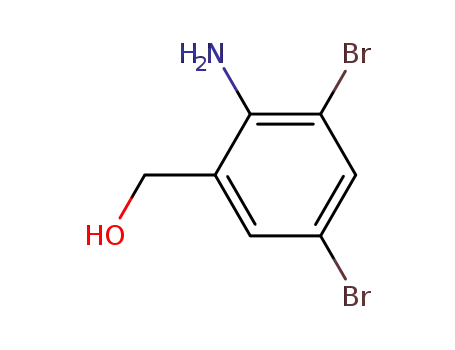
- 50739-76-9
(2-amino-3,5-dibromophenyl)methanol

-
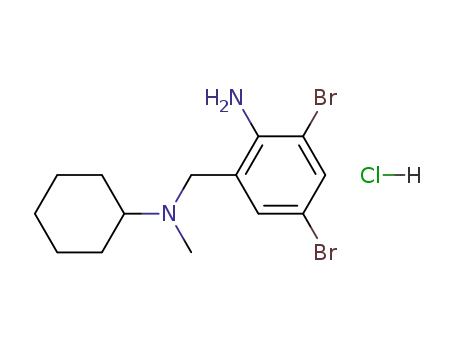
- 611-75-6
bromhexine monohydrochloride
| Conditions | Yield |
|---|---|
|
N-methylcyclohexylamine; (2-amino-3,5-dibromophenyl)methanol; With bis(trichloromethyl) carbonate; sodium hydrogencarbonate; In tetrahydrofuran; at 0 - 20 ℃; for 2h;
With hydrogenchloride; In ethanol;
|
95% |
|
N-methylcyclohexylamine; (2-amino-3,5-dibromophenyl)methanol; With acetic acid; In toluene; at 85 ℃; for 12h; Industrial scale;
With hydrogenchloride; In acetone; at 20 ℃; for 1.33333h; pH=2 - 3; Industrial scale;
|
80% |
|
N-methylcyclohexylamine; (2-amino-3,5-dibromophenyl)methanol; With acetic acid; In toluene; at 143 - 178 ℃; for 30h;
With hydrogenchloride; In water; ethyl acetate; toluene; at 0 - 10 ℃; Reagent/catalyst; Temperature;
|
-
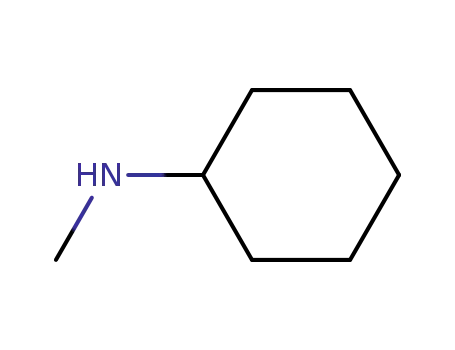
- 100-60-7
N-methylcyclohexylamine

-
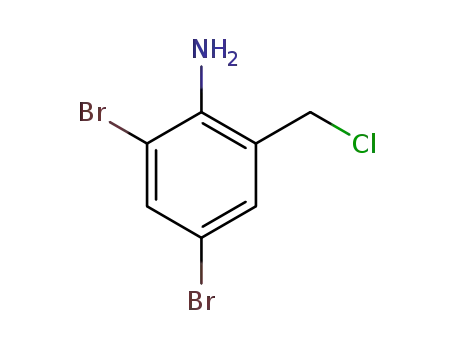
-
2-chloromethyl-4,6-dibromoaniline

-

- 611-75-6
bromhexine monohydrochloride
| Conditions | Yield |
|---|---|
|
at 45 - 55 ℃; for 3h;
|
89.8% |
|
N-methylcyclohexylamine; 2-chloromethyl-4,6-dibromoaniline; With pyrographite; In ethanol; at 20 - 30 ℃; for 1h; Heating;
With hydrogenchloride; In ethanol; at 0 - 10 ℃; for 0.5h; pH=4 - Ca. 5; Temperature; pH-value; Solvent; Heating;
|
63.25% |
|
N-methylcyclohexylamine; 2-chloromethyl-4,6-dibromoaniline; In dichloromethane; at 0 - 5 ℃; for 3h;
With hydrogenchloride; In ethanol; dichloromethane; at 25 - 30 ℃;
|
59.6% |
611-75-6 Upstream products
-
100-60-7
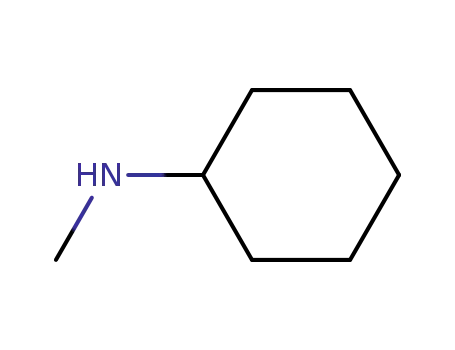
N-methylcyclohexylamine
-
50739-76-9

(2-amino-3,5-dibromophenyl)methanol
-
63498-16-8
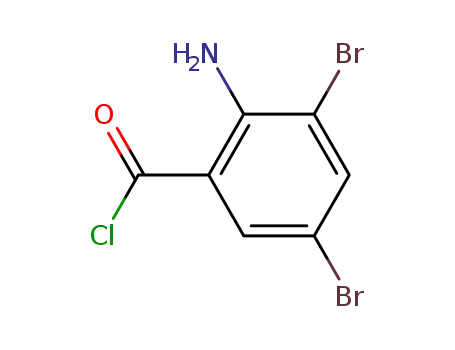
2-Amino-3,5-dibromo-benzoyl chloride
-
50910-55-9
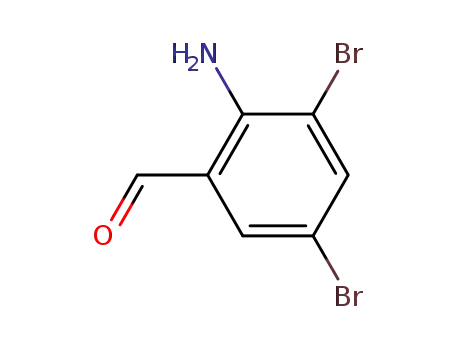
3,5-dibromo-2-amino benzaldehyde
611-75-6 Downstream products
-
5432-28-0
N-nitroso-N-methylcyclohexylamine
-
50910-55-9

3,5-dibromo-2-amino benzaldehyde
-
114390-40-8
3-Cyclohexyl-6,8-dibromchinazolin-4-on
-
114390-43-1
SF-150B(1)
Relevant Products
-
1,3-Dibromo-5,5-dimethylhydantoin
CAS:77-48-5
-
Rutile
CAS:1317-80-2
-
Liquid Red Mercury
CAS:20720-76-7