68131-73-7
- Product Name:Polyethylene-polyamines
- Molecular Formula:C12H5N7O12
- Purity:99%
- Molecular Weight:0
Product Details;
CasNo: 68131-73-7
Molecular Formula: C12H5N7O12
Appearance: orange-red to brown viscous liquid
Quality Manufacturer Supply High Purity 99% Polyethylene-polyamines 68131-73-7 Low Price
- Molecular Formula:C12H5N7O12
- Molecular Weight:0
- Appearance/Colour:orange-red to brown viscous liquid
- Vapor Pressure:0.001Pa at 20℃
- Boiling Point:443℃[at 101 325 Pa]
- PKA:9.21[at 20 ℃]
- PSA:0.00000
- Density:1.014[at 20℃]
- LogP:0.00000
Polyethylene-polyamines(Cas 68131-73-7) Usage
Polyethylene polyamines are liquid chemicals characterized by a yellowish color and an amine odor. They exhibit miscibility in water and possess the ability to neutralize acids, forming salts and water. These chemicals, which include diethylenetriamine (DETA), triethylenetetramine (TETA), tetraethylenepentamine (TEPA), and pentaethylenehexamine (PEHA), can react with strong reducing agents, producing flammable gaseous hydrogen.
In terms of compatibility, polyethylene polyamines may be incompatible with various substances such as isocyanates, halogenated organics, peroxides, acidic phenols, epoxides, anhydrides, and acid halides. Notably, they have the potential to neutralize acids in exothermic reactions.
Polyamines, a broader class of molecules, are involved in modulating RNA enzyme activities and are synthesized from amino acids like arginine, ornithine, and methionine. Specific polyethylene polyamines, like triethylenetetramine (TETA) and diethylenetriamine (DETA), have been studied for their protective properties on sulfidic ores. When used alone in dilute solutions or combined with potassium amyl xanthate (p.a.x.), they form an effective protective layer on sulfidic ores, significantly reducing oxidation caused by atmospheric oxygen. Experimental studies have demonstrated their efficacy in preventing oxidation in both small particles and coarse samples when exposed to diluted hydrogen peroxide solutions or atmospheric oxygen as oxidants.
68131-73-7 Relevant articles
Preventing oxidation of iron sulfide minerals by polyethylene polyamines
Yu-Wei Chen a, Yuerong Li a 1, Mei-Fang Cai a b, Nelson Belzile a, Zhi Dang b
, Minerals Engineering Volume 19, Issue 1, January 2006, Pages 19-27
The purpose of this work was thus to investigate whether polyethylene polyamines alone or combinations of this group of chemicals with p.a.x. could produce an effective coating layer to prevent the fast oxidation occurring at the surface of sulfidic ores, namely pyrite and pyrrhotite.
Comparative study of polyethylene polyamines as activator molecules for a structurally unstable group I ribozyme
Mst. Ara Gulshan,Shigeyoshi Matsumura,Tsunehiko Higuchi,Naoki Umezawa &Yoshiya Ikawa
, Bioscience, Biotechnology, and Biochemistry Volume 82, 2018 - Issue 8
To systematically analyze the effects of amine moieties in polyamines, we used four polyethylene polyamines (Figure 1(D)). We performed cleavage reactions (Figure 1(B)) in the presence of equimolar amounts of the substrate RNA and the ∆P5 ribozyme [Citation9].
Relevant Products
-
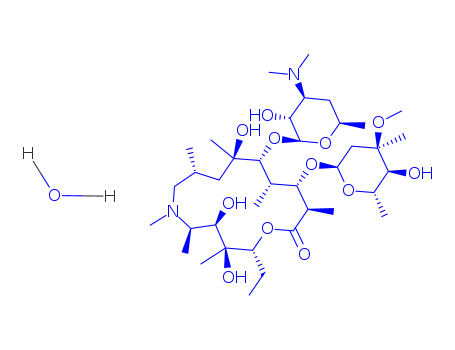
Azithromycin dihydrate
CAS:117772-70-0
-
coixan
CAS:102483-16-9
-
MOLECULAR SIEVE
CAS:1344-00-9