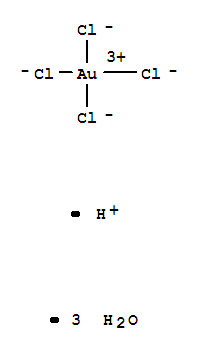
1318-93-0
- Product Name:K-CATALYST
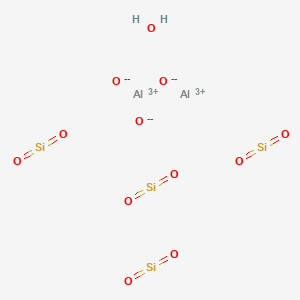
- Molecular Formula:Al2O9Si3
- Purity:99%
- Molecular Weight:30.06900
Product Details;
CasNo: 1318-93-0
Molecular Formula: Al2O9Si3
Buy High Quality K-CATALYST, Sale 1318-93-0 Fast Shipping
- Molecular Formula:Al2O9Si3
- Molecular Weight:30.06900
- PSA:0.00000
- Density:2-3
- LogP:1.02620
K-CATALYST (Cas 1318-93-0) Usage
K-CATALYST is a natural clay mineral belonging to the smectite group, characterized by its dioctahedral 2:1 phyllosilicate structure with two tetrahedral sheets and one octahedral sheet. Named after the French village of Montmorillon, it forms microscopic crystals and has unique properties.
Being a 2:1 clay mineral, K-CATALYST exhibits high water absorption capacity and significant expansion when exposed to water. It can also swell in water and has a notable cation-exchange capacity. This mineral is utilized in various applications, including industrial protective creams, calamine lotion, wet compresses, anti-irritants for eczema, mud packs, sunburn paint, baby and face powders, and face creams.
In industrial uses, K-CATALYST is employed as a component of foundry sand and as a desiccant for moisture removal from air and gases. It has also been extensively used in catalytic processes, particularly in cracking catalysts for over 60 years, and in other acid-based catalysts.
1318-93-0 Relevant articles
Preparation and properties of montmorillonite modified asphalts
Jianying Yu, Xuan Zeng, Shaopeng Wu, Lin Wang, Gang Liu
, Materials Science and Engineering: A Volume 447, Issues 1–2, 25 February 2007, Pages 233-238
Compared with MMT, OMMT showed better effect in improving softening point and rutting resistance of asphalt, which contributes to the formation of exfoliated structure in OMMT modified asphalt. Storage stability tests disclose that the asphalts modified with MMT or OMMT are very stable when montmorillonite content is less than 3 wt%.
Low temperature carbon particulate oxidation on a supported Cu/V/K catalyst
P. Ciambelli a, V. Palma a, S. Vaccaro b
, Catalysis Today Volume 17, Issues 1–2, 26 May 1993, Pages 71-78
Catalyst characterization suggested that a redox mechanism is responsible for the strong activity of the catalyst. Carbon oxides TPD measurements gave evidence that the energy of desorption of the carbon-oxygen surface complexes is dramatically modified by the presence of catalyst.
Relevant Products
-
HYDROGEN TETRACHLOROAURATE(III)
CAS:16961-25-4
-
Sucrose
CAS:57-50-1
-
Cellulose acetate
CAS:9004-35-7