13951-70-7
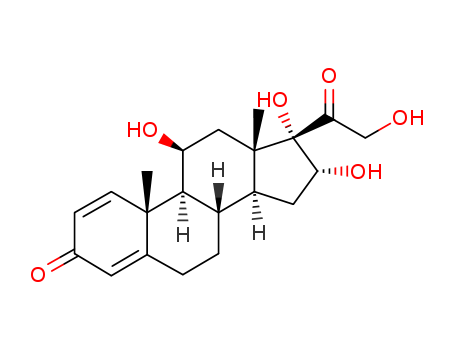
- Product Name:11a,16b,17,21-Tetrahydroxy-pregna-1,4-diene-3,20-dione
- Molecular Formula:C21H28O6
- Purity:99%
- Molecular Weight:376.45
Product Details;
CasNo: 13951-70-7
Molecular Formula: C21H28O6
Appearance: white crystalline solid
Wholesale 11a,16b,17,21-Tetrahydroxy-pregna-1,4-diene-3,20-dione 13951-70-7 Efficient Shipping Low Price
- Molecular Formula:C21H28O6
- Molecular Weight:376.45
- Appearance/Colour:white crystalline solid
- Vapor Pressure:1.86E-16mmHg at 25°C
- Melting Point:235-238 °C
- Refractive Index:1.629
- Boiling Point:591.5 °C at 760 mmHg
- PKA:11.93±0.70(Predicted)
- Flash Point:325.6 °C
- PSA:115.06000
- Density:1.381 g/cm3
- LogP:0.52840
11a,16b,17,21-Tetrahydroxy-pregna-1,4-diene-3,20-dione(Cas 13951-70-7) Usage
|
Chemical Properties |
11α,16β,17,21-Tetrahydroxy-pregna-1,4-diene-3,20-dione, also known as 16α-hydroxyprednisolone, is a steroid compound with a complex molecular structure. It is characterized by the presence of four hydroxy (-OH) groups at specific positions on the steroid nucleus, along with double bonds at positions 1 and 4, and ketone groups at positions 3 and 20. |
|
Uses |
Pharmaceutical Applications: 11α,16β,17,21-Tetrahydroxy-pregna-1,4-diene-3,20-dione finds extensive use in the pharmaceutical industry, particularly in the synthesis of corticosteroids and related medications. It serves as a key intermediate in the production of various corticosteroid drugs used for their anti-inflammatory, immunosuppressive, and metabolic effects. Anti-inflammatory Medications: This compound, also known as 16α-hydroxyprednisolone, is utilized in the synthesis of medications with potent anti-inflammatory properties. These medications are often prescribed for the treatment of inflammatory conditions such as arthritis, asthma, dermatitis, and allergic reactions. Immunosuppressive Agents: Certain corticosteroid drugs derived from 11α,16β,17,21-Tetrahydroxy-pregna-1,4-diene-3,20-dione are employed as immunosuppressive agents in the management of autoimmune disorders and to prevent organ rejection in transplant recipients. Dermatological Treatments: Some formulations containing corticosteroids synthesized from this compound are utilized topically in dermatology for the treatment of various skin conditions, including eczema, psoriasis, and allergic dermatitis. Research: In addition to its pharmaceutical applications, 11α,16β,17,21-Tetrahydroxy-pregna-1,4-diene-3,20-dione is also used as a research tool in studies investigating the mechanisms of action of corticosteroids and their potential therapeutic applications in various disease states. |
InChI:InChI=1/C21H28O6/c1-19-6-5-12(23)7-11(19)3-4-13-14-8-16(25)21(27,17(26)10-22)20(14,2)9-15(24)18(13)19/h5-7,13-16,18,22,24-25,27H,3-4,8-10H2,1-2H3
13951-70-7 Relevant articles
Preparation method and application of 16, 17-dihydroxy steroid compound
-
, (2022/01/08)
The invention provides a preparation met...
Method for preparing 16 alpha-hydroxyprednisolone
-
, (2020/07/02)
The invention discloses a method for pre...
Preparation method of high-purity 16 alpha-hydroxyprednisolone
-
Paragraph 0026-0040, (2021/01/04)
The invention discloses a preparation me...
9-site dehalogenation method of steroid compound
-
Paragraph 0020, (2020/05/01)
The invention discloses a 9-site dehalog...
13951-70-7 Process route
-
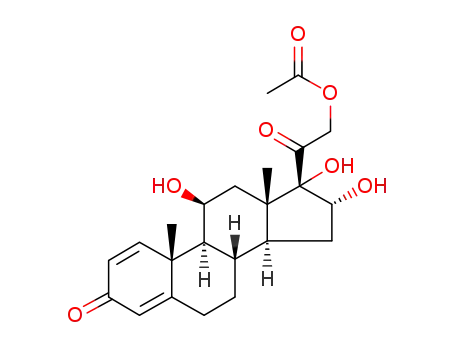
- 86401-80-1
11β,16α,17α,21-tetrahydroxypregnane-1,4-diene-3,20-dione-21-acetate

-
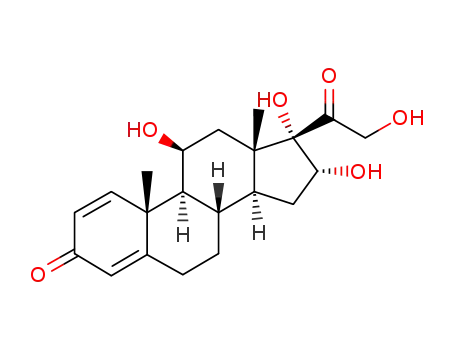
- 13951-70-7
desonide
| Conditions | Yield |
|---|---|
|
With sodium hydroxide; In methanol; dichloromethane; at 0 - 5 ℃; for 1h; Inert atmosphere;
|
93.5% |
|
With sodium hydroxide; In methanol; at 20 ℃; for 2h;
|
91.1% |
|
In toluene; at 40 - 45 ℃; Solvent;
|
78.9% |
|
With sodium sulfite; In methanol; dichloromethane; water; at 0 ℃; Reagent/catalyst;
|
73% |
|
With methanol; potassium hydroxide; In ethanol; dichloromethane; at -10 ℃; Reagent/catalyst; Temperature;
|
36 mg |
-
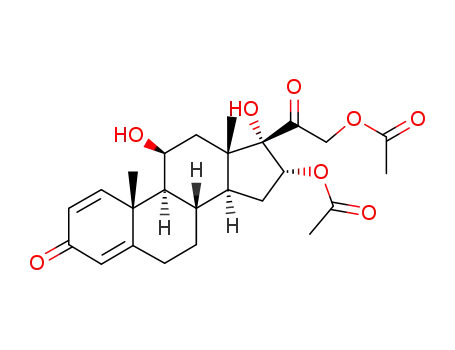
- 98422-55-0
16α-21-diacetoxyprednisolone

-

- 13951-70-7
desonide
| Conditions | Yield |
|---|---|
|
In toluene; at 40 - 45 ℃; Solvent;
|
70.5% |
|
In toluene; at 40 - 45 ℃; Solvent;
|
70.5% |
|
In toluene; at 40 - 45 ℃; Solvent;
|
70.5% |
|
With solid phase base catalyst; In toluene; at 40 - 45 ℃;
|
|
|
With solid phase alkali catalyst; In toluene; at 40 - 45 ℃; Solvent; Reagent/catalyst;
|
70.5 g |
13951-70-7 Upstream products
-
1171-81-9
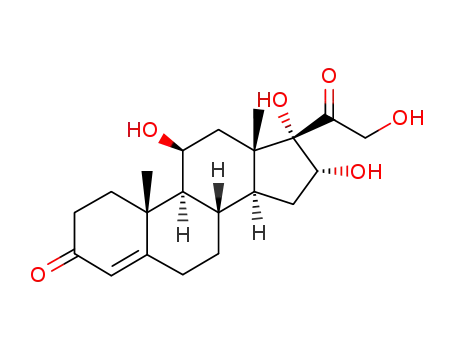
11β,16α,17,21-tetrahydroxy-pregn-4-ene-3,20-dione
-
37413-91-5
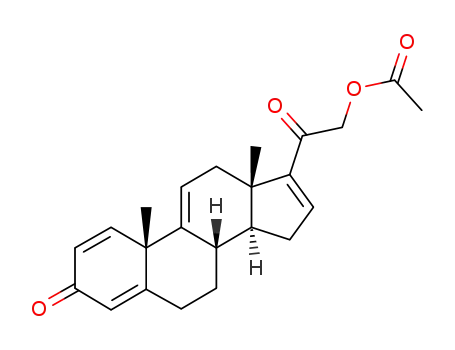
2-((10S,13S,14S)-10,13-dimethyl-3-oxo-6,7,8,10,12,13,14,15-octahydro-3H-cyclopenta[a]phenanthren-17-yl)-2-oxoethyl acetate
-
77017-20-0
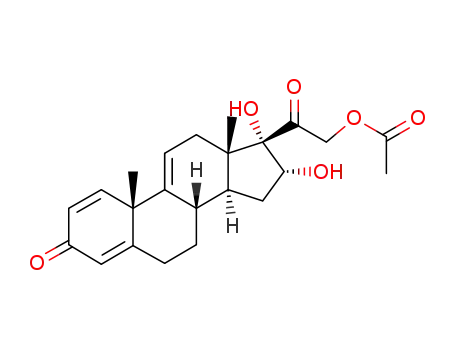
16α,17α,21-trihydroxypregna-1,4,9(11)-triene-3,20-dione-21-acetate
-
91160-89-3
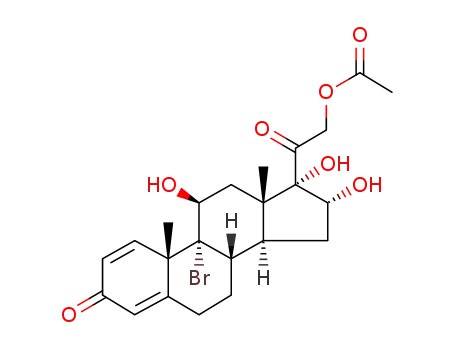
9α-bromo-11β,16α,17α,21-tetrahydroxypregnane-1,4-diene-3,20-dione-21-acetate
13951-70-7 Downstream products
-
638-94-8
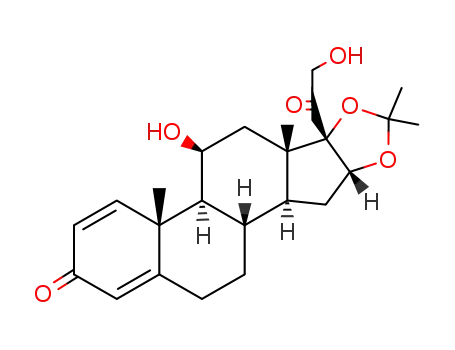
desonide
-
124-94-7
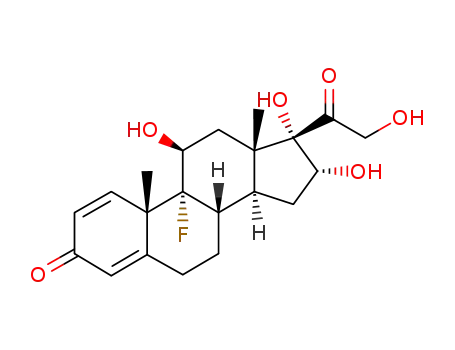
triamcinolone
-
22199-85-5
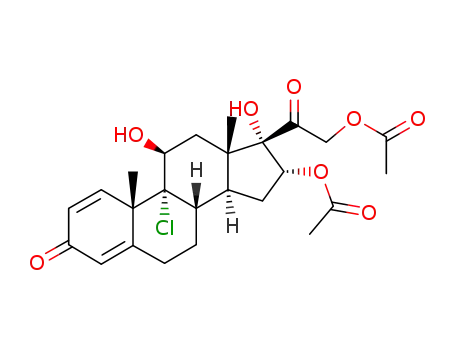
16α,21-diacetoxy-9-chloro-11β,17-dihydroxy-pregna-1,4-diene-3,20-dione
-
1868-22-0
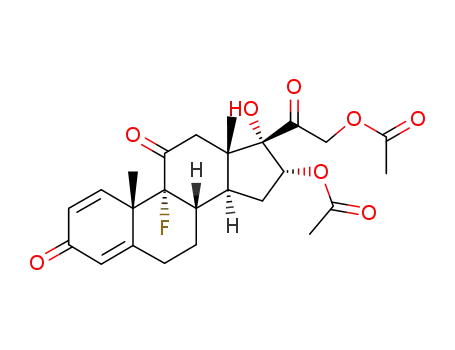
16α,21-diacetoxy-9-fluoro-17-hydroxy-pregna-1,4-diene-3,11,20-trione
Relevant Products
-
Azithromycin dihydrate
CAS:117772-70-0
-
Oxytocin
CAS:50-56-6
-
Melanotan II
CAS:121062-08-6