120511-73-1
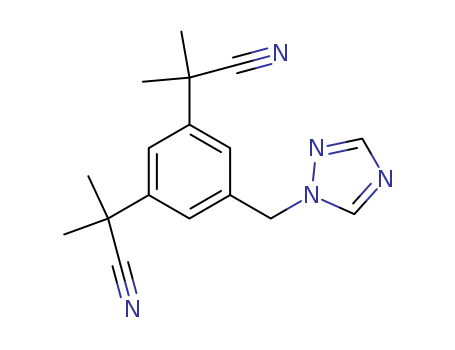
- Product Name:Anastrozole
- Molecular Formula:C17H19N5
- Purity:99%
- Molecular Weight:293.371
Product Details;
CasNo: 120511-73-1
Molecular Formula: C17H19N5
Appearance: crystalline solid
99% Pure Factory Supply High Purity Anastrozole 120511-73-1 Reasonable Price
- Molecular Formula:C17H19N5
- Molecular Weight:293.371
- Appearance/Colour:crystalline solid
- Melting Point:81 - 82 °C
- Refractive Index:1.791
- Boiling Point:469.718 °C at 760 mmHg
- PKA:2.62±0.10(Predicted)
- Flash Point:237.877 °C
- PSA:78.29000
- Density:1.085 g/cm3
- LogP:2.92876
Anastrozole(Cas 120511-73-1) Usage
|
Description |
Anastrozole is a hormone treatment medication known for lowering estrogen levels in the body, particularly in postmenopausal women with hormone-dependent breast cancer. Chemically identified as 2,2-[5-(1H-1,2,4-triazol-1-ylmethyl)-1,3-phenylene]bis(2-methylpropiononitrile), it is a third-generation aromatase inhibitor with the trade name ARIMIDEX®. |
|
Uses |
Widely used in breast cancer treatment, Anastrozole is indicated for early-stage, hormone receptor-positive breast cancer in postmenopausal women. It has shown effectiveness in reducing the risks of breast cancer recurrence and metastasis when compared to tamoxifen. The medication is well-tolerated, with the primary adverse effect being an increased risk of bone fractures during the treatment period. Anastrozole has linear pharmacokinetics, is metabolized in the liver, and is unlikely to cause clinically significant drug interactions. |
|
Function |
Functioning as a competitive and selective inhibitor of aromatase, Anastrozole prevents the conversion of testosterone into estradiol and androstenedione into estrone. This inhibition occurs through competitive binding of aromatase to the hemegroup of cytochrome P450, reducing estrogen biosynthesis in peripheral tissues and the breast. |
| Synthesis |
The synthesis of Anastrozole involves a four-step process, and it has been used in various research applications. Classified as readily biodegradable and moderately mobile in soils, Anastrozole is a potent and highly selective aromatase inhibitor used as second-line therapy in postmenopausal breast cancer treatment after tamoxifen. |
|
Chemical Properties |
Crystalline Solid. soluble in organic solvents such as ethanol, DMSO, and dimethyl formamide. The solubility of anastrozole in these solvents is approximately 20, 13, and 14 mg/ml. |
|
Originator |
Zeneca (United Kingdom) |
|
Definition |
ChEBI: Anastrozole is a 1,2,4-triazole compound having a 3,5-bis(2-cyano-2-propyl)benzyl group at the 1-position. It has a role as an antineoplastic agent and an EC 1.14.14.14 (aromatase) inhibitor. It is a member of triazoles and a nitrile. |
|
Brand name |
Arimidex (AstraZeneca). |
|
Therapeutic Function |
Antitumor |
|
Biological Activity |
Potent and highly selective aromatase (CYP19) inhibitor (IC 50 = 15nM) that has no discernible effect on adrenocorticoid hormone synthesis. Reduces plasma estrogen levels and exhibits antitumor activity in vivo . Orally active. |
|
Biochem/physiol Actions |
Anastrozole, which contains a triazole functional group, reversibly binds to the cytochrome P-450 component of aromatase. Binding interferes with the catalytic properties of aromatase, which results in inhibition of estrogen synthesis. |
|
Drug interactions |
Potentially hazardous interactions with other drugs Oestrogen-containing therapies: avoid concomitant administration as would negate pharmacological action. Tamoxifen: avoid concomitant administration. |
InChI:InChI=1S/C17H19N5/c1-16(2,9-18)14-5-13(8-22-12-20-11-21-22)6-15(7-14)17(3,4)10-19/h5-7,11-12H,8H2,1-4H3
120511-73-1 Relevant articles
How to use the Lasentec FBRM probe on manufacturing scale
Adlington, Neil K.,Black, Simon N,Adshead, David L
, p. 557 - 567 (2013)
A Lasentec FBRM probe was installed in a...
Pharmacological and clinical profile of anastrozole
Per E. Lønning, Jürgen Geisler & Mitch Dowsett
Breast Cancer Research and Treatment, Volume 49, pages S53–S57, (1998)
While follow-up results have not revealed any significant difference in time to relapse between the drug regimens, they have revealed an improved survival among patients treated with anastrozole 1 mg compared to megestrol acetate 160 mg daily.
Use of anastrozole for breast cancer prevention (IBIS-II): long-term results of a randomised controlled trial
Prof Jack Cuzick, PhD Ivana Sestak, PhD Prof John F Forbes, MD Prof Mitch Dowsett, PhD
The Lancet, 2020
3864 women were recruited between Feb 2, 2003, and Jan 31, 2012. 1920 women were randomly assigned to 5 years anastrozole and 1944 to placebo. After a median follow-up of 131 months (IQR 105–156), a 49% reduction in breast cancer was observed for anastrozole (85 vs 165 cases, hazard ratio [HR] 0·51, 95% CI 0·39–0·66, p<0·0001).
METHOD FOR PREPARING ANASTROZOLE FOR PHARMACEUTICAL PURPOSES
-
Page/Page column 4; 5; 6, (2014/12/12)
A method for preparing anastrozole chara...
120511-73-1 Process route
-
![2-[3-bromomethyl-5-(cyanodimethylmethyl)phenyl]-2-methylpropanenitrile](/upload/2024/1/2584575d-33c1-4a94-a62e-f92a332bdf8e.png)
- 120511-84-4
2-[3-bromomethyl-5-(cyanodimethylmethyl)phenyl]-2-methylpropanenitrile

-
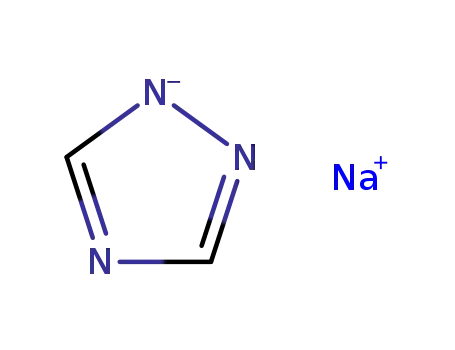
- 41253-21-8
sodium triazole

-
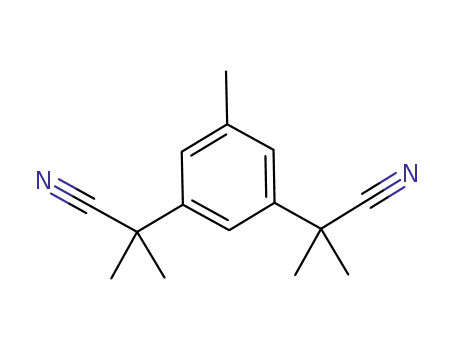
- 120511-72-0
3,5-bis(2-cyanoprop-2-yl)toluene

-
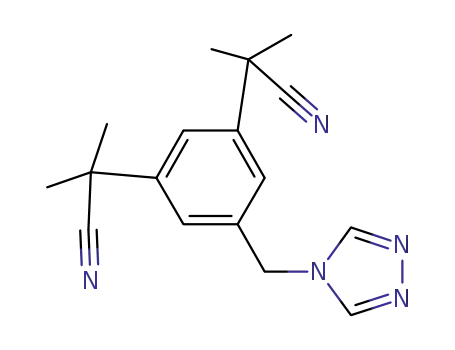
- 120511-92-4
α,α,α',α'-tetramethyl-5-(4H-1,2,4-triazol-4ylmethyl)-1,3-benzene-diacetonitrile

-
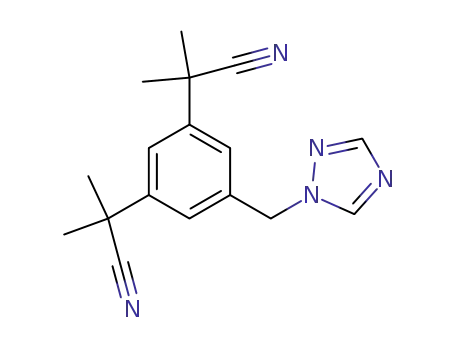
- 120511-73-1
anastrozol
| Conditions | Yield |
|---|---|
|
In N,N-dimethyl-formamide; at -10 - -5 ℃; for 0.75h; Product distribution / selectivity;
|

-

- 120511-72-0
3,5-bis(2-cyanoprop-2-yl)toluene

-

- 120511-92-4
α,α,α',α'-tetramethyl-5-(4H-1,2,4-triazol-4ylmethyl)-1,3-benzene-diacetonitrile

-

- 120511-73-1
anastrozol
| Conditions | Yield |
|---|---|
|
In di-isopropyl ether; toluene; at 30 - 35 ℃; for 0.5 - 2h; Purification / work up; Heating / reflux;
|
120511-73-1 Upstream products
-
120511-91-3
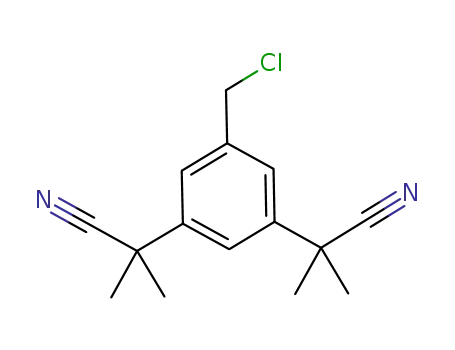
α,α,α',α'-tetramethyl-5-chloromethyl-1,3-benzene diacetonitrile
-
1015083-81-4
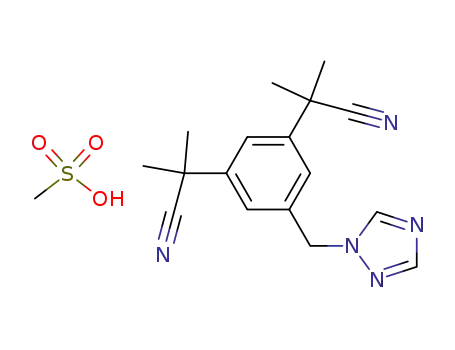
anastrozole mesylate
-
120511-84-4
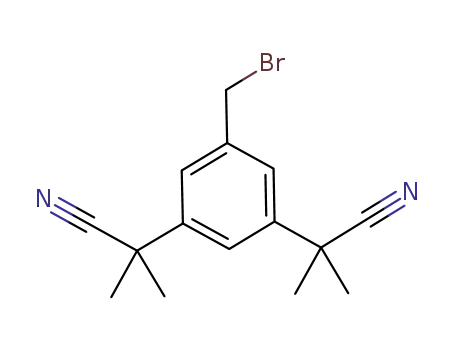
2-[3-bromomethyl-5-(cyanodimethylmethyl)phenyl]-2-methylpropanenitrile
-
41253-21-8

sodium triazole
120511-73-1 Downstream products
-
1015083-81-4

anastrozole mesylate
-
120511-72-0

3,5-bis(2-cyanoprop-2-yl)toluene
Relevant Products
-
Azithromycin dihydrate
CAS:117772-70-0
-
Lactose
CAS:63-42-3
-
Vanillin
CAS:121-33-5