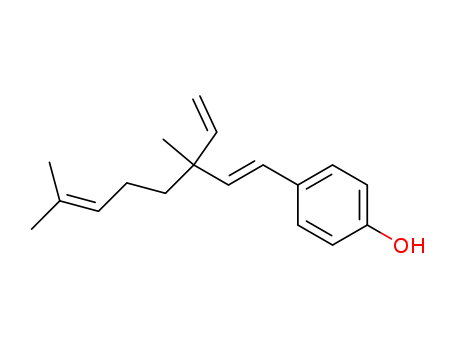
10309-37-2
- Product Name:Bakuchiol
- Molecular Formula:C18H24 O
- Purity:99%
- Molecular Weight:256.388
Product Details;
CasNo: 10309-37-2
Molecular Formula: C18H24 O
Appearance: white-off solid or colorless liquid
Buy Quality Wholesale Bakuchiol 10309-37-2 Low Price
- Molecular Formula:C18H24O
- Molecular Weight:256.388
- Appearance/Colour:white-off solid or colorless liquid
- Vapor Pressure:1.1E-06mmHg at 25°C
- Refractive Index:1.554
- Boiling Point:391.4°Cat760mmHg
- PKA:10.10±0.26(Predicted)
- Flash Point:176.6°C
- PSA:20.23000
- Density:0.963g/cm3
- LogP:5.34410
Bakuchiol(Cas 10309-37-2) Usage
|
Description |
Bakuchiol, extracted from the seeds and leaves of the babchi plant (Psoralea corylifolia), is a plant-based compound with a history of use in traditional Chinese and Indian Ayurvedic medicine. It shares skin benefits with retinol but is reputed to lack its side effects. The compound is known to stimulate collagen production, reduce fine lines and wrinkles, improve skin texture and radiance, and even out skin tone. Users may experience initial improvements within 4 to 6 weeks, with more pronounced results, such as improved wrinkles, firmness, and pigmentation, requiring longer-term use. |
|
Uses |
Studies indicate that Bakuchiol, specifically the (S)-Bakuchiol variant found in Psoralea glandulosa, exhibits antifungal and anti-tumor effects. It has been identified as a potent cytotoxic agent with concentration-dependent growth inhibition against leukemia cancer cells. Bakuchiol, with the chemical formula [(1E,3S)-3-ethenyl-3,7-dimethyl-1,6-octadien-1-yl]phenol, belongs to the Leguminosae plant family. Research has demonstrated its various pharmacological effects, including antioxidant, antibacterial, anti-inflammatory, antiaging, and estrogen-like properties. The compound offers protective effects in the heart, liver, skin, and other organs. It protects against ischemia-reperfusion injury in the heart, inhibits liver fibrosis, and has anticancer effects on various cancer cells. Bakuchiol also slows skin aging by preserving collagen, protects against bone loss, and reduces blood glucose and triglycerides, showing potential in protecting against pancreatic beta-cell damage and diabetes progression. The review emphasizes the pharmacological mechanisms and protective effects of Bakuchiol in human diseases, with a focus on its impact on the heart, liver, and other vital organs. |
|
Safety Profile |
Poison by intravenous andintraperitoneal routes. Moderately toxic by ingestion.When heated to decomposition it emits acrid smoke andfumes. |
InChI:InChI=1/C18H24O/c1-5-18(4,13-6-7-15(2)3)14-12-16-8-10-17(19)11-9-16/h5,7-12,14,19H,1,6,13H2,2-4H3/b14-12+/t18-/m1/s1
10309-37-2 Relevant articles
Bakuchiol: A newly discovered warrior against organ damage
Zhenlong Xin a b 1, Xue Wu a 1, Ting Ji a 1, Baoping Xu a, Yuehu Han c, Meng Sun d, Shuai Jiang e, Tian Li b, Wei Hu b, Chao Deng f, Yang Yang a
, Pharmacological Research Volume 141, March 2019, Pages 208-213
Psoralea corylifolia is a member of the Leguminosae plant family and a traditional Chinese medicine [1]; previous research indicates that Psoralea corylifolia contains multiple chemical substances, such as bakuchiol (BAK), psoralen, psoralidin, isopsoralen, and bavachin...
Enantioselective Synthesis of Quaternary Stereocenters via Chromium Catalysis
Xiong, Yang,Zhang, Guozhu
supporting information, p. 5094 - 5097 (2016/10/14)
Asymmetric allylation of aldehydes with ...
A facile asymmetric synthesis of Δ3-2-Hydroxybakuchiol, Bakuchiol and ent-Bakuchiol
Xu, Qian-Qian,Zhao, Qun,Shan, Guang-Sheng,Yang, Xi-Cheng,Shi, Qi-Yuan,Lei, Xinsheng
, p. 10739 - 10746 (2013/12/04)
A facile asymmetric synthesis of Δ3-2-Hy...
10309-37-2 Process route
-
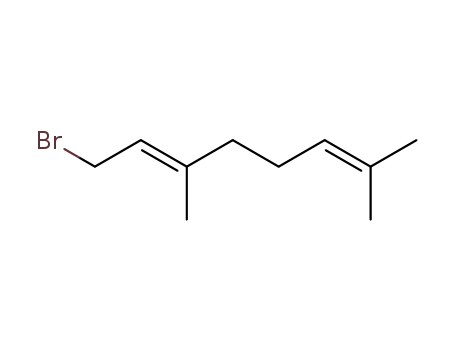
- 6138-90-5
trans-geranyl bromide

-
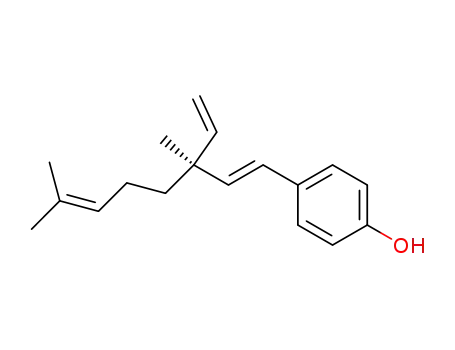
- 17015-60-0,93998-10-8,10309-37-2
bakuchiol
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 3 steps
1.1: 3,4,5-trifluoro-N-(2-((S)-4-isopropyl-4,5-dihydrooxazol-2-yl)-6-(((1S,2R,5S)-2-isopropyl-5-methylcyclohexyl)oxy)phenyl)benzenesulfonamide; chromium dichloride; zirconocene dichloride; cesium iodide; nickel dichloride; manganese / tetrahydrofuran / 10 h / -10 °C / Inert atmosphere
2.1: pyridine; methanesulfonyl chloride / 10 h / 20 °C
2.2: 5 h / 20 °C
3.1: methyl magnesium iodide / 0.17 h / 185 °C / Inert atmosphere
With pyridine; chromium dichloride; manganese; methyl magnesium iodide; zirconocene dichloride; 3,4,5-trifluoro-N-(2-((S)-4-isopropyl-4,5-dihydrooxazol-2-yl)-6-(((1S,2R,5S)-2-isopropyl-5-methylcyclohexyl)oxy)phenyl)benzenesulfonamide; methanesulfonyl chloride; nickel dichloride; cesium iodide; In tetrahydrofuran;
|
-
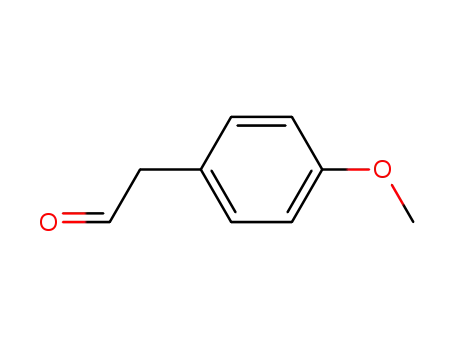
- 5703-26-4
4-Methoxyphenylacetaldehyde

-

- 17015-60-0,93998-10-8,10309-37-2
bakuchiol
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 3 steps
1.1: 3,4,5-trifluoro-N-(2-((S)-4-isopropyl-4,5-dihydrooxazol-2-yl)-6-(((1S,2R,5S)-2-isopropyl-5-methylcyclohexyl)oxy)phenyl)benzenesulfonamide; chromium dichloride; zirconocene dichloride; cesium iodide; nickel dichloride; manganese / tetrahydrofuran / 10 h / -10 °C / Inert atmosphere
2.1: pyridine; methanesulfonyl chloride / 10 h / 20 °C
2.2: 5 h / 20 °C
3.1: methyl magnesium iodide / 0.17 h / 185 °C / Inert atmosphere
With pyridine; chromium dichloride; manganese; methyl magnesium iodide; zirconocene dichloride; 3,4,5-trifluoro-N-(2-((S)-4-isopropyl-4,5-dihydrooxazol-2-yl)-6-(((1S,2R,5S)-2-isopropyl-5-methylcyclohexyl)oxy)phenyl)benzenesulfonamide; methanesulfonyl chloride; nickel dichloride; cesium iodide; In tetrahydrofuran;
|
10309-37-2 Upstream products
-
17015-63-3

(+/-)-bakuchiol methyl ether
-
110-93-0
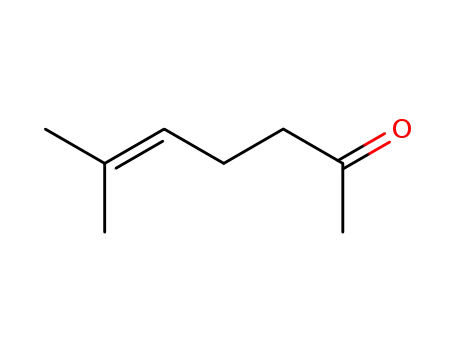
6-Methyl-hept-5-en-2-on
-
104846-78-8
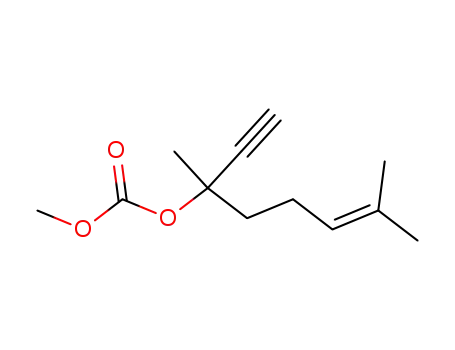
3,7-dimethyl-oct-6-en-1-yn-3-yl methyl carbonate
-
106-24-1

Geraniol
10309-37-2 Downstream products
-
43010-52-2
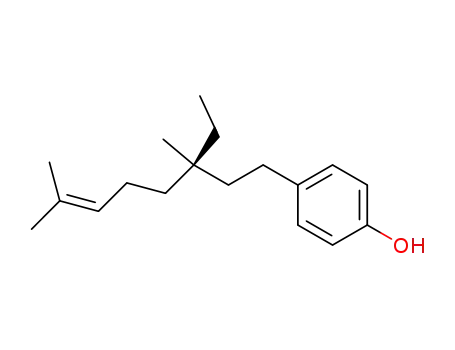
7,8,16,17-tetrahydrobakuchiol
-
43010-50-0
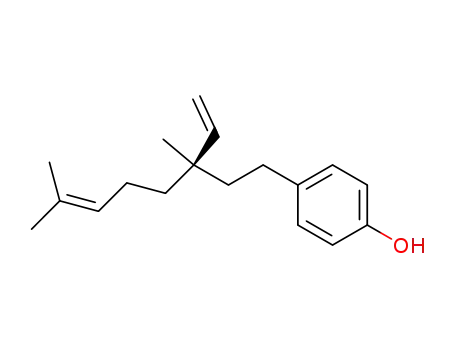
7,8-dihydrobakuchiol
-
10309-38-3
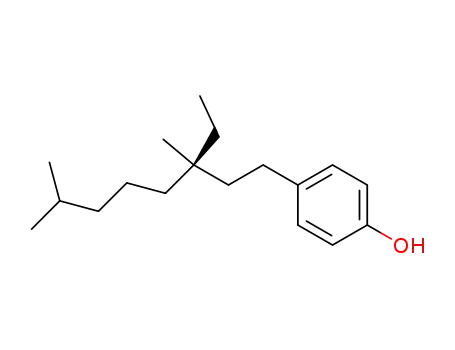
4-(3-ethyl-3,7-dimethyloctyl)phenol
-
43010-46-4
(S,E)-4-(3,7-dimethyl-3-vinylocta-1,6-dien-1-yl)phenyl acetate
Relevant Products
-
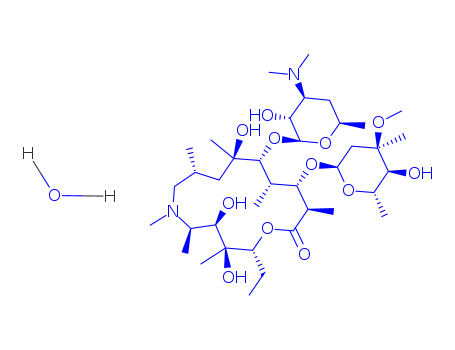
Azithromycin dihydrate
CAS:117772-70-0
-
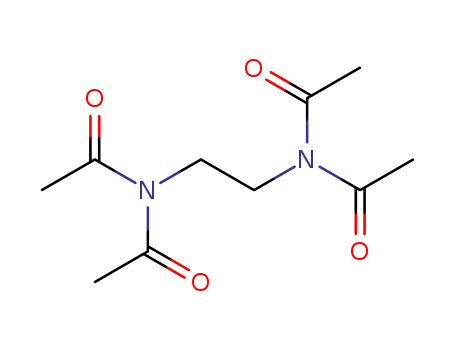
Tetraacetylethylenediamine
CAS:10543-57-4
-
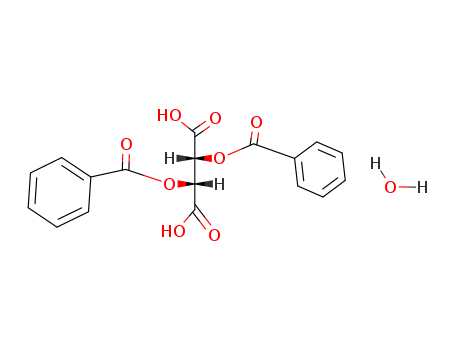
(-)-Dibenzoyl-L-tartaric acid monohydrate
CAS:62708-56-9