537-73-5
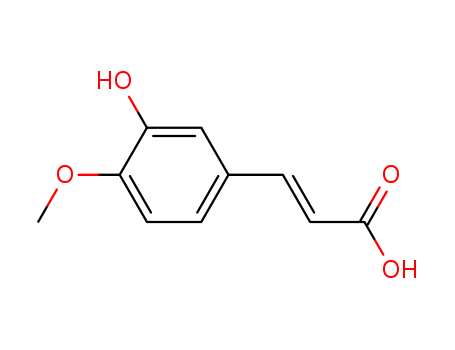
- Product Name:3-Hydroxy-4-methoxycinnamic acid
- Molecular Formula:C10H10O4
- Purity:99%
- Molecular Weight:194.187
Product Details;
CasNo: 537-73-5
Molecular Formula: C10H10O4
Appearance: Off-white crystalline solid
Chinese Manufacturer Supply High Purity 99% 3-Hydroxy-4-methoxycinnamic acid 537-73-5 with Best Price
- Molecular Formula:C10H10O4
- Molecular Weight:194.187
- Appearance/Colour:Off-white crystalline solid
- Vapor Pressure:1.83E-07mmHg at 25°C
- Melting Point:230 °C (dec.)(lit.)
- Refractive Index:1.5168 (estimate)
- Boiling Point:410.2 °C at 760 mmHg
- PKA:4.53±0.10(Predicted)
- Flash Point:167.6 °C
- PSA:66.76000
- Density:1.316 g/cm3
- LogP:1.49860
3-Hydroxy-4-methoxycinnamic acid(Cas 537-73-5) Usage
|
Chemical Properties |
Off-White Crystalline Solid |
|
Uses |
3-Hydroxy-4-methoxycinnamic acid serves as an efficient acetylcholine inhibitor and is a bioactive metabolite of Spilanthes acmella Murr. It plays a role in increasing the resistance of low-density lipoprotein (LDL) to oxidation. Beyond its natural occurrence, 3-Hydroxy-4-methoxycinnamic acid is recognized for its antidiabetic activity. It is found in various organisms, including Sibiraea angustata, Astragalus onobrychis, black cohosh, and Ipomoea aquatica leaves. This compound showcases potential therapeutic properties, particularly in the context of managing diabetes and oxidative stress. |
|
Definition |
ChEBI: A ferulic acid consisting of trans-cinnamic acid bearing methoxy and hydroxy substituents at positions 4 and 3 respectively on the phenyl ring. |
|
General Description |
3-Hydroxy-4-methoxycinnamic acid, commonly known as Isoferulic acid, is a hypoglycemic agent available in the form of white crystals. It is isolated from the aerial part of Artemisia capillaris, a Chinese medicinal plant. |
InChI:InChI=1/C10H10O4/c1-14-9-4-2-7(6-8(9)11)3-5-10(12)13/h2-6,11H,1H3,(H,12,13)/p-1/b5-3+
537-73-5 Relevant articles
Insulin-Releasing Properties of a Series of Cinnamic Acid Derivatives in Vitro and in Vivo
S Adisakwattana, P Moonsan
, J. Agric. Food Chem. 2008, 56, 17, 7838–7844
For example, isoferulic acid (3-hydroxy-4-methoxycinnamic acid) exhibits significantly decreased levels of plasma glucose concentration in streptozotocin (STZ)-induced diabetic rats by …
Isoferuloyl derivatives of five seed glucosinolates in the crucifer genus Barbarea
Agerbirk, Niels,Olsen, Carl Erik
, p. 610 - 623 (2011)
Five acylated glucosinolates (GSLs) were...
Regioselectivity of Cobalamin-Dependent Methyltransferase Can Be Tuned by Reaction Conditions and Substrate
Pompei, Simona,Grimm, Christopher,Farnberger, Judith E.,Schober, Lukas,Kroutil, Wolfgang
, p. 5977 - 5983 (2020/10/06)
Regioselective reactions represent a sig...
Copper and L-(?)-quebrachitol catalyzed hydroxylation and amination of aryl halides under air
Bao, Xuefei,Chen, Guoliang,Dong, Jinhua,Du, Fangyu,Li, Hui,Liang, Xinjie,Wu, Ying,Zhang, Yongsheng
supporting information, (2020/08/03)
L-(?)-Quebrachitol, a natural product ob...
Structural features and antioxidant activities of Chinese quince (Chaenomeles sinensis) fruits lignin during auto-catalyzed ethanol organosolv pretreatment
Cheng, Xi-Chuang,Guo, Xin-Ran,Liu, Hua-Min,Liu, Yu-Lan,Qin, Zhao,Wang, Xue-De
, p. 4348 - 4358 (2020/09/22)
Chinese quince fruits (Chaenomeles sinen...
537-73-5 Process route
-
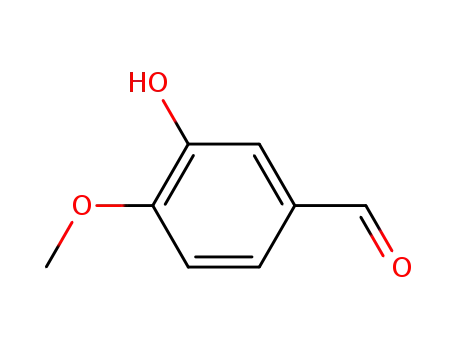
- 621-59-0
isovanillin

-
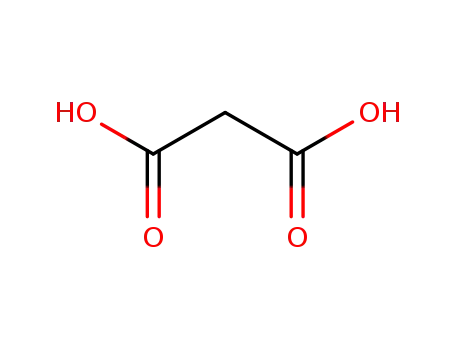
- 141-82-2
malonic acid

-
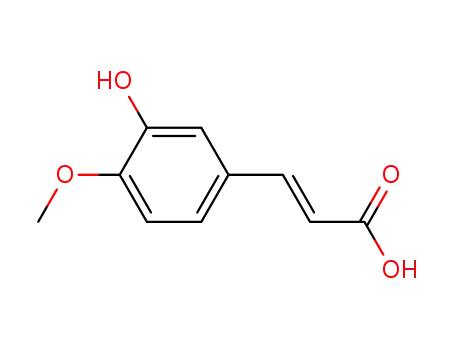
- 537-73-5
trans-3-hydroxy-4-methoxycinnamic acid
| Conditions | Yield |
|---|---|
|
With piperidine; pyridine; for 3h; Heating;
|
94% |
|
With piperidine; pyridine; In ethanol; for 3h; Heating;
|
92% |
|
With pyridine; acetic acid; at 130 ℃; for 0.333333h; microwave irradiation;
|
86% |
|
With N,N-dimethyl acetamide; triethylamine; N,N-dimethyl-formamide; In toluene; for 4h; Reflux;
|
85% |
|
With piperidine; pyridine; In toluene; at 110 ℃; for 12h; Dean-Stark;
|
78.4% |
|
With pyridine; 1,8-diazabicyclo[5.4.0]undec-7-ene; for 4h; Reflux; Inert atmosphere;
|
77.2% |
|
With piperidine; pyridine; at 120 ℃; for 4h;
|
58% |
|
With piperidine; pyridine; for 3.5h; Heating;
|
52% |
|
With piperidine; pyridine;
|
|
|
With piperidine; In pyridine;
|
|
|
In pyridine; for 3h; Heating;
|
|
|
With piperidine; pyridine; at 90 ℃;
|
|
|
isovanillin; malonic acid; With piperidine; pyridine; at 120 ℃; for 6h;
With hydrogenchloride; In water; pH=3;
|
|
|
With piperidine; In pyridine; at 120 ℃; for 1.5h;
|
|
|
With pyridine; at 90 ℃;
|
|
|
With pyridine; aniline; In toluene; at 95 ℃;
|
-
- 103261-25-2
3-iodo-4-methoxy-trans-cinnamic acid

-

- 537-73-5
trans-3-hydroxy-4-methoxycinnamic acid
| Conditions | Yield |
|---|---|
|
With copper(I) oxide; 1-D-O-Methyl-chiro-inositol; sodium hydroxide; In water; at 120 ℃; for 6h;
|
81% |
537-73-5 Upstream products
-
621-59-0

isovanillin
-
141-82-2

malonic acid
-
58435-27-1
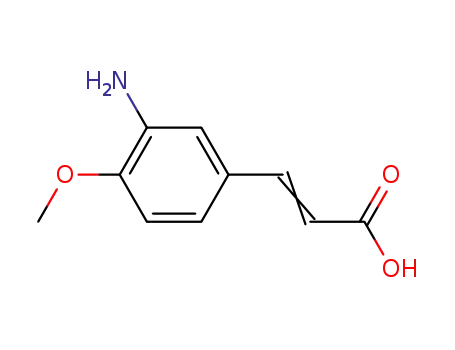
3-amino-4-methoxy-cinnamic acid
-
97966-29-5
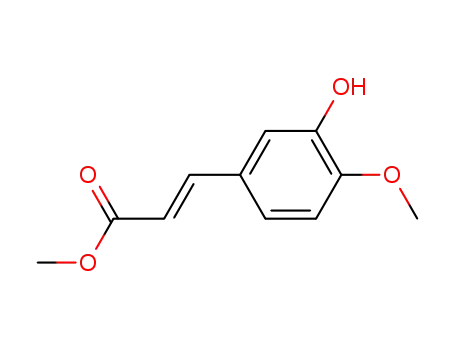
methyl (E)-3-(3-hydroxy-4-methoxyphenyl)-2-propenoate
537-73-5 Downstream products
-
97966-29-5

methyl (E)-3-(3-hydroxy-4-methoxyphenyl)-2-propenoate
-
1135-15-5
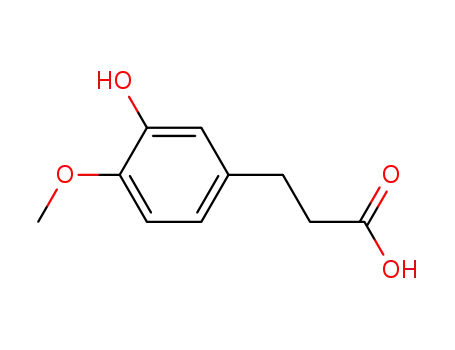
dihydroisoferulic acid
-
3207-31-6
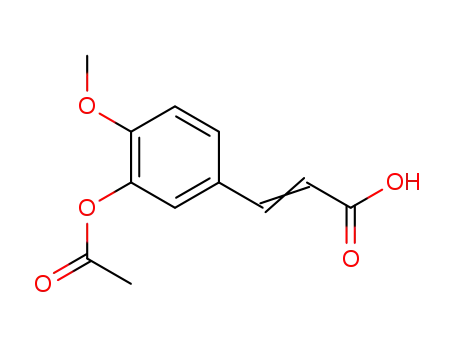
3-(3-acetoxy-4-methoxyphenyl)acrylic acid
-
30461-77-9
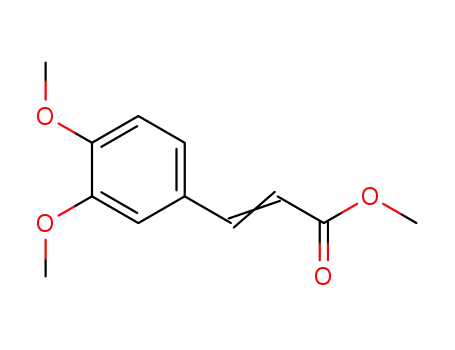
methyl 3,4-dimethoxycinnamate
Relevant Products
-
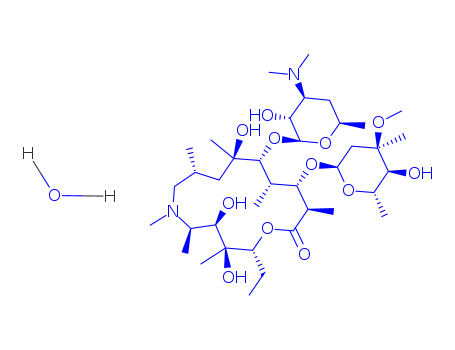
Azithromycin dihydrate
CAS:117772-70-0
-
Carboxymethyl cellulose
CAS:9000-11-7
-
D(+)-GLUCOSE MONOHYDRATE
CAS:14431-43-7