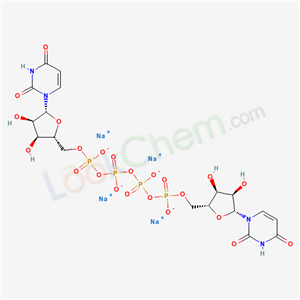
211427-08-6
- Product Name:INS 365
- Molecular Formula:C18H22N4Na4O23P4
- Purity:99%
- Molecular Weight:878.2344
Product Details;
CasNo: 211427-08-6
Molecular Formula: C18H22N4Na4O23P4
Factory Supply High Purity 99% INS 365 211427-08-6 On Stock
- Molecular Formula:C18H22N4Na4O23P4
- Molecular Weight:878.2344
- Boiling Point:°Cat760mmHg
- Flash Point:°C
- PSA:455.01000
- Density:g/cm3
- LogP:-2.43740
INS 365(Cas 211427-08-6) Usage
|
Description |
Diquafosol tetrasodium (INS-365) is a pharmaceutical drug used for the treatment of dry eye disease. It is formulated as a 3% ophthalmic solution of the tetrasodium salt. Diquafosol acts as a P2Y2 receptor agonist, stimulating the secretion of fluids and mucin on the ocular surface. |
|
Originator |
Inspire Pharmaceuticals (United States) |
|
Uses |
Dry Eye Disease Treatment: Diquafosol is primarily used as a topical treatment for dry eye disease. It is effective in increasing tear fluid secretion, improving corneal epithelial resistance, and enhancing the release of glycoproteins on the ocular surface. Specific Dry Eye Disorders: Diquafosol has shown effectiveness in treating various types of dry eye disorders, including aqueous-deficient dry eye, short tear film break-up time dry eye, obstructive meibomian gland dysfunction, dry eye following laser in situ keratomileusis (LASIK) surgery, and dry eye following cataract surgery. |
|
Brand name |
Diquas |
| Metabolism | Upon administration, diquafosol is metabolized by phosphodiesterases to various metabolites, including UTP, UDP, UMP, and uridine. These metabolites may contribute to its pharmacological effects on the ocular surface. |
InChI:InChI=1/C18H26N4O23P4.4Na/c23-9-1-3-21(17(29)19-9)15-13(27)11(25)7(41-15)5-39-46(31,32)43-48(35,36)45-49(37,38)44-47(33,34)40-6-8-12(26)14(28)16(42-8)22-4-2-10(24)20-18(22)30;;;;/h1-4,7-8,11-16,25-28H,5-6H2,(H,31,32)(H,33,34)(H,35,36)(H,37,38)(H,19,23,29)(H,20,24,30);;;;/q;4*+1/p-4/t7-,8-,11-,12-,13-,14-,15-,16-;;;;/m1..../s1
211427-08-6 Relevant articles
Safety of aerosolized INS 365 in patients with mild to moderate cystic fibrosis: Results of a phase I multi-center study
Peadar G. Noone MD, Nicole Hamblett PhD, Frank Accurso MD, Moira L. Aitken MD, Michael Boyle MD, Mark Dovey MD, Ron Gibson MD, PhD, Craig Johnson MS, Don Kellerman PhD, Michael W. Konstan MD, Laura Milgram MD, Jean Mundahl MS, George Retsch-Bogort MD, David Rodman MD, Judy Williams-Warren MPH, RN, Robert W. Wilmott MD, Pam Zeitlin MD, Bonnie Ramsey MD
, Pediatric Pulmonology, Volume32, Issue2 August 2001 Pages 122-128
Triphosphate nucleotides such as uridine-5′-triphosphate (UTP) and INS 365, may be useful for CF through actions, mediated via P2Y2 extracellular receptors, on chloride and liquid secretion, and ciliary beat frequency. INS 365 may offer chemical stability advantages over UTP.
Efficacy and Safety of Diquafosol Ophthalmic Solution in Patients with Dry Eye Syndrome: A Japanese Phase 2 Clinical Trial
Yukihiro Matsumoto MD 1, Yuichi Ohashi MD 2, Hitoshi Watanabe MD 3, Kazuo Tsubota MD 1, Diquafosol Ophthalmic Solution Phase 2 Study Group ⁎
Ophthalmology Volume 119, Issue 10, October 2012, Pages 1954-1960
Rose Bengal corneal and conjunctival staining scores also improved significantly with both 1% and 3% diquafosol compared with placebo (P = 0.007 and P = 0.004, respectively). Subjective dry eye symptom scores significantly improved with both diquafosol ophthalmic solutions (P ≤ 0.033), although there were no significant differences in BUT compared with placebo. No significant differences between the treatment groups were observed in relation to the occurrence of adverse events.
Relevant Products
-
Monosodium glutamate
CAS:32221-81-1
-
Vanillin
CAS:121-33-5
-
Kojic acid
CAS:501-30-4