554-13-2
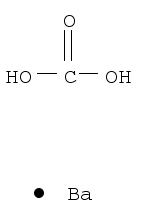
- Product Name:Lithium carbonate
- Molecular Formula:Li2CO3
- Purity:99%
- Molecular Weight:73.8912
Product Details;
CasNo: 554-13-2
Molecular Formula: Li2CO3
Appearance: white fine crystalline powder
Quality Factory Supply Lithium carbonate ,Sale 554-13-2 Safe Delivery
- Molecular Formula:Li2CO3
- Molecular Weight:73.8912
- Appearance/Colour:white fine crystalline powder
- Melting Point:720 °C
- Boiling Point:333.6 °C at 760 mmHg
- Flash Point:169.8 °C
- PSA:63.19000
- Density:2.11 g/cm3
- LogP:-2.44700
Lithium carbonate(Cas 554-13-2) Usage
|
General Description |
Lithium carbonate is a medication used to treat and prevent episodes of mania (abnormally elevated mood) in people with bipolar disorder. It works by reducing the activity of certain chemicals in the brain, including serotonin and dopamine. It is also used to reduce the frequency and severity of manic episodes in patients with bipolar disorder. Additionally, lithium carbonate is known to help reduce the risk of suicide in people with mood disorders. This medication is typically taken in capsule or tablet form and is usually prescribed alongside other treatments, such as therapy and other medications. It is important to use lithium carbonate as directed by a healthcare professional, as improper use can lead to serious side effects and complications. |
InChI:InChI=1/CH2O3.2Li/c2-1(3)4;;/h(H2,2,3,4);;/q;2*+1/p-2
554-13-2 Relevant articles
Changes of nitrides characteristics in Li-N system synthesized at different pressures
Ignatenko, Oleg V.,Komar, Valery A.,Leonchik, Sergey V.,Shempel, Natalia A.,Ene, Antoaneta,Cantaragiu, Alina,Frontasyeva, Marina V.,Shvetsov, Valery N.
, p. 23 - 27 (2013)
For the Li-N system samples were obtaine...
On the incompatibility of lithium-O2 battery technology with CO2
Zhang, Shiyu,Nava, Matthew J.,Chow, Gary K.,Lopez, Nazario,Wu, Gang,Britt, David R.,Nocera, Daniel G.,Cummins, Christopher C.
, p. 6117 - 6122 (2017)
When solubilized in a hexacarboxamide cr...
A study of binary iron/lithium organometallic complexes as single source precursors to solid state cathode materials for potential Li ion battery application
Khanderi, Jayaprakash,Schneider, J?rg J.
, p. 254 - 259 (2011)
Solid state and solution phase decomposi...
Synthesis of Li4SiO4 by a modified combustion method
Cruz, Daniel,Bulbulian, Silvia
, p. 1720 - 1724 (2005)
We report for the first time the synthes...
Characterization of Li1-xNi1+xO2 prepared using succinic acid as a complexing agent
Thongtem, Titipun,Thongtem, Somchai
, p. 202 - 209 (2006)
Li1-xNi1+xO2 was prepared by a polymeriz...
Lithium silicate nanosheets with excellent capture capacity and kinetics with unprecedented stability for high-temperature CO2capture
Belgamwar, Rajesh,Maity, Ayan,Das, Tisita,Chakraborty, Sudip,Vinod, Chathakudath P.,Polshettiwar, Vivek
, p. 4825 - 4835 (2021)
An excessive amount of CO2is the leading...
Synthesis and CO2 adsorption characteristics of lithium zirconates with high lithia content
Yin, Xian-Sheng,Li, Shao-Peng,Zhang, Qin-Hui,Yu, Jian-Guo
, p. 2837 - 2842 (2010)
Pure monoclinic phase Li6Zr2O7 and rhomb...
Electrochemical Decomposition of Li4SiO4 and Li2TiO3 in Solid-state Thermal Cells
Aceves, Juan M.,West, Anthony R.
, p. 2599 - 2608 (1982)
Cells of the type Au/Li4SiO4/Au and Au/L...
Evidence of CO2 chemisorption at high temperature in lithium Gallate (Li5GaO4)
Avalos-Rendon, Tatiana,Pfeiffer, Heriberto
, p. 504 - 505 (2011)
Li5GaO4 was tested as a possibleCO2 capt...
Thermally driven interfacial degradation between Li7La3Zr2O12 electrolyte and LiNi0.6Mn0.2Co0.2O2 cathode
Kim, Younggyu,Kim, Dongha,Bliem, Roland,Vardar, Gülin,Waluyo, Iradwikanari,Hunt, Adrian,Wright, Joshua T.,Katsoudas, John P.,Yildiz, Bilge
, p. 9531 - 9541 (2020)
Solid-state batteries offer higher energ...
Adsorption and electrodeposition on SnO2 and WO3 electrodes in 1 M LiClO4/PC. In situ light scattering and in situ atomic force microscopy studies
Isidorsson,Lindstroem,Granqvist,Herranen
, p. 2784 - 2795 (2000)
A novel spectroscopic in situ light scat...
Time-Resolved Synchrotron Powder X-ray Diffraction Studies on the Synthesis of Li8SiO6 and Its Reaction with CO2
Cova, Federico,Amica, Guillermina,Kohop??, Katja,Blanco, Maria Valeria
, p. 1040 - 1047 (2019)
Lithium oxosilicate was synthesized via ...
Three-step calcination synthesis of high-purity Li8ZrO 6 with CO2 absorption properties
Yin, Xian-Sheng,Zhang, Qin-Hui,Yu, Jian-Guo
, p. 2844 - 2850 (2011)
Li8ZrO6 contains a high lithium content ...
Dependence of electrochemical properties of spinel LiMn2O4 on Li2CO3 with micro-flaky, micro-flower and nanorod morphologies
Li, Lang,Sui, Jinsong,Huang, Rui,Xiang, Wei,Qin, Wei
, p. 42289 - 42295 (2017)
Herein, the dependence of spinel LiMn2O4...
REMARKS ON THE BINARY SYSTEMS Li//2O-Me//2O//5 (Me equals Nb,Ta).
Abbattista, F.,Vallino, M.,Mazza, D.
, p. 1019 - 1028 (1987)
In the Li//2O-Ta//2O//5 system there are...
Selective production of acetone in the electrochemical reduction of CO2 catalyzed by a Ru-naphthyridine complex
Mizukawa, Tetsunori,Tsuge, Kiyoshi,Nakajima, Hiroshi,Tanaka, Koji
, p. 362 - 363 (1999)
The controlled potential electrolysis of...
Study of the phases in a copper cathode during an electrodeposition process for obtaining Cu-Li alloys
Lambri,Perez-Landazabal,Penaloza,Herrero,Recarte,Ortiz,Woerner
, p. 1023 - 1033 (2000)
The evolution of Cu-18at%Li crystals, Cu...
Reaction mechanisms of Li0.30La0.57TiO3 powder with ambient air: H+/Li+ exchange with water and Li2CO3 formation
Boulant, Anthony,Bardeau, Jean Francois,Jouanneaux, Alain,Emery, Joel,Buzare, Jean-Yves,Bohnke, Odile
, p. 3968 - 3975 (2010)
The proton/lithium exchange property of ...
Mechanism of a reversible CO2 capture monitored by the layered perovskite Li2SrTa2O7
Galven, Cyrille,Fourquet, Jean-Louis,Suard, Emmanuelle,Crosnier-Lopez, Marie-Pierre,Le Berre, Franoise
, p. 4191 - 4197 (2010)
We demonstrate for the first time, a new...
Colloidal-crystal-templated synthesis of ordered macroporous electrode materials for lithium secondary batteries
Yan, Hongwei,Sokolov, Sergey,Lytle, Justin C.,Stein, Andreas,Zhang, Fan,Smyrl, William H.
, p. A1102-A1107 (2003)
This paper presents a general method of ...
A Biomimetic Nickel Complex with a Reduced CO2 Ligand Generated by Formate Deprotonation and Its Behaviour towards CO2
Zimmermann, Philipp,Hoof, Santina,Braun-Cula, Beatrice,Herwig, Christian,Limberg, Christian
, (2018)
Reduced CO2 species are key intermediate...
Selective CO2 Splitting by Doubly Reduced Aryl Boranes to Give CO and [CO3]2?
von Grotthuss, Esther,Prey, Sven E.,Bolte, Michael,Lerner, Hans-Wolfram,Wagner, Matthias
, p. 16491 - 16495 (2018)
Alkali metal salts M2[1] (M=Li, Na) of d...
Effect of excess Li+in solution on LiFePO4preparation via wet chemical method
He, Lihua,Zhao, Zhongwei
, p. 386 - 391 (2016)
Olivine structure LiFePO4has attracted m...
MOSSBAUER STUDY OF THE THERMAL DECOMPOSITION OF ALKALI TRIS(OXALATO)FERRATES(III).
Brar,Randhawa
, p. 153 - 156 (1985)
The thermal decomposition of alkali (Li,...
Gel-combustion synthesis of Li1.2Mn0.4Co 0.4O2 composites with a high capacity and superior rate capability for lithium-ion batteries
Fu, Chaochao,Li, Guangshe,Luo, Dong,Zheng, Jing,Li, Liping
, p. 1471 - 1483 (2014)
Owing to the merits of high capacity and...
H2O-induced self-propagating synthesis of hierarchical porous carbon: A promising lithium storage material with superior rate capability and ultra-long cycling life
Liang, Chu,Liang, Sheng,Xia, Yang,Chen, Yun,Huang, Hui,Gan, Yongping,Tao, Xinyong,Zhang, Jun,Zhang, Wenkui
, p. 18221 - 18229 (2017)
Hierarchical porous carbon (HPC) has att...
Features of the Thermolysis of Li, Na, and Cd Maleates
Avdin, V. V.,Merzlov, S. V.,Nayfert, S. A.,Polozov, M. A.,Polozova, V. V.,Sakthi Dharan, C. P.,Taskaev, S. V.,Zherebtsov, D. A.
, p. 1311 - 1318 (2020/07/21)
Abstract: Processes of the multi-stage d...
The Alkali Metal Salts of Methyl Xanthic Acid
Liebing, Phil,Schmeide, Marten,Kühling, Marcel,Witzorke, Juliane
, p. 2428 - 2434 (2020/06/17)
Methyl xanthates of the type M(SSC-OMe) ...
554-13-2 Process route
-

-
lithium carbonyl

-
- 1333-74-0
hydrogen

-
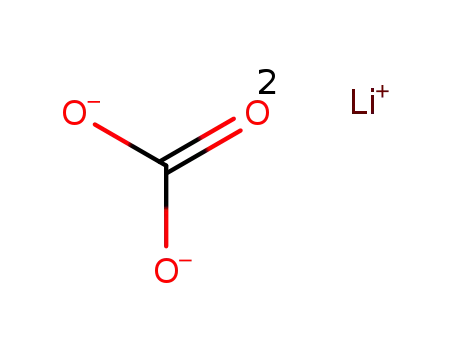
- 554-13-2
lithium carbonate

-
- 7440-44-0
pyrographite
| Conditions | Yield |
|---|---|
|
With H2O; In water; on treating with water vigorous explosion with ignition of the gaseous products;;
|
-
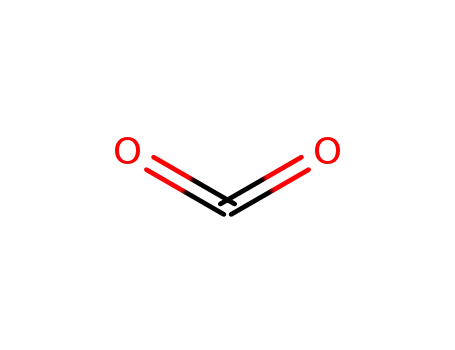
- 124-38-9,18923-20-1
carbon dioxide

-
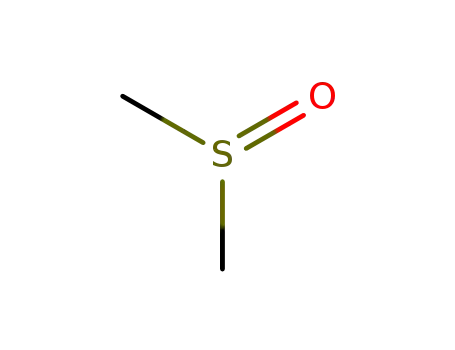
- 67-68-5,8070-53-9
dimethyl sulfoxide

-
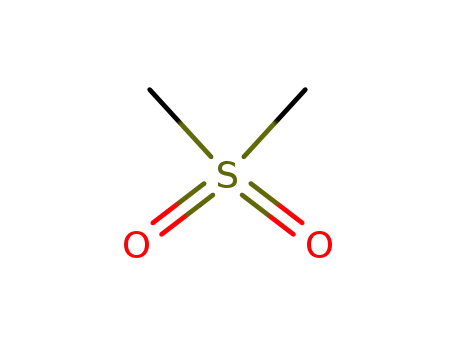
- 67-71-0
dimethylsulfone

-

- 554-13-2
lithium carbonate
| Conditions | Yield |
|---|---|
|
With lithium peroxide; at 25 ℃; for 48h; under 760.051 Torr;
|
90 %Spectr. |
554-13-2 Upstream products
-
584-08-7
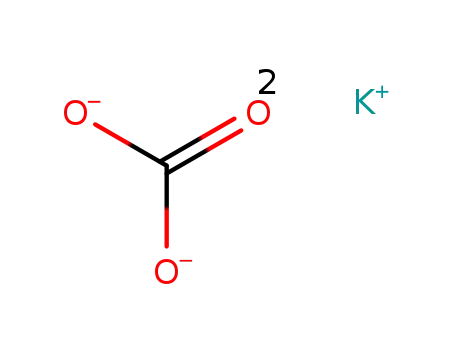
potassium carbonate
-
6108-17-4
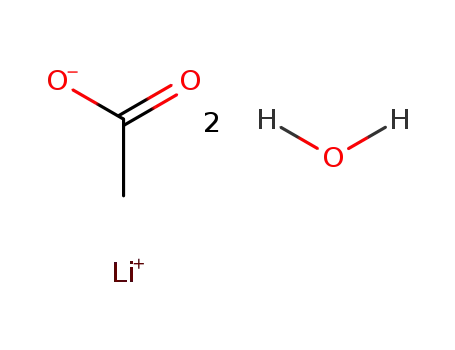
lithium acetate dihydrate
-
6018-89-9
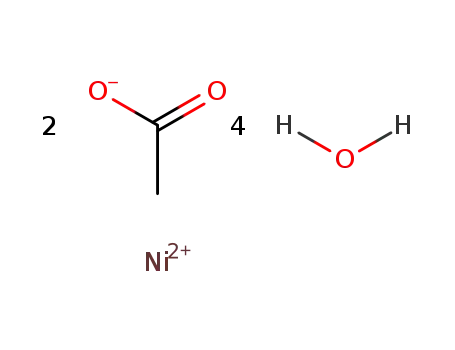
nickel(II) acetate tetrahydrate
-
1310-66-3
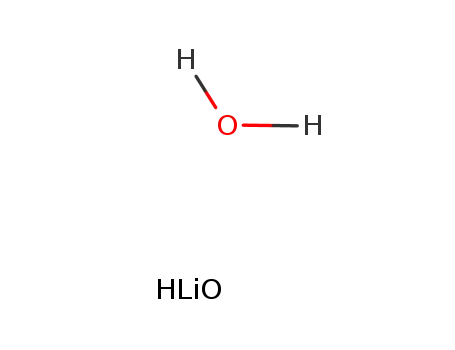
lithium hydroxide monohydrate
554-13-2 Downstream products
-
57272-09-0
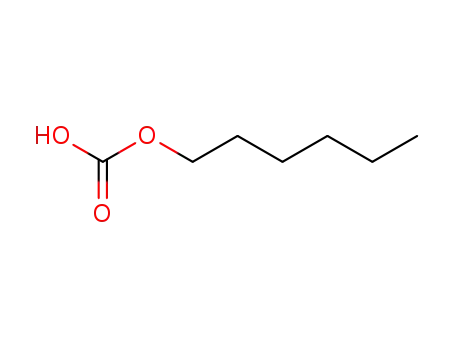
hexylcarbonate
-
67399-93-3
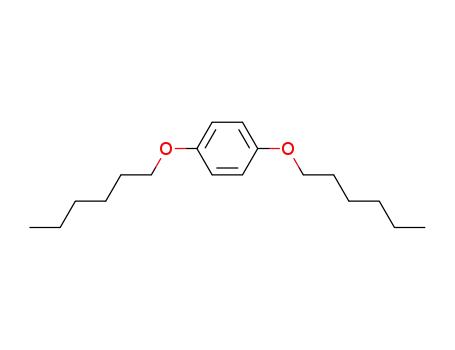
1,4-dihexyloxybenzene
-
18979-55-0
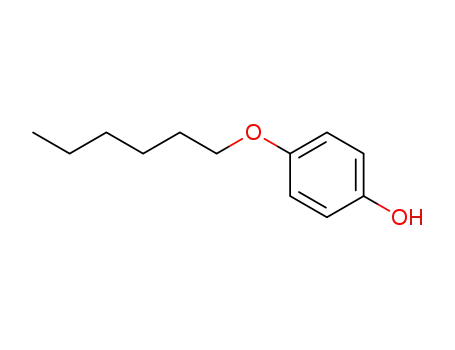
4-(hexyloxy)phenol
-
107835-88-1
(RS)-3-benzyl-5-(hydroxymethyl)-1,3-oxazolidin-2-one
Relevant Products
-
Human serum albumin
CAS:70024-90-7
-
Liquid Red Mercury
CAS:20720-76-7
-
Barium carbonate
CAS:513-77-9