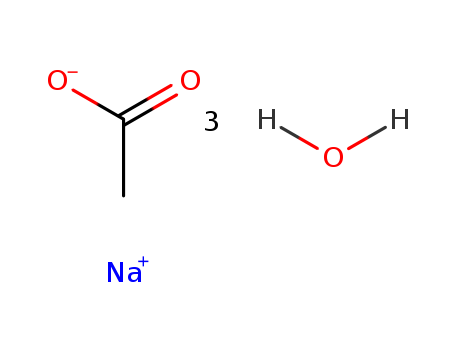
6131-90-4
- Product Name:Sodium acetate trihydrate
- Molecular Formula:C2H3NaO2.3(H2O)
- Purity:99%
- Molecular Weight:136.08
Product Details;
CasNo: 6131-90-4
Molecular Formula: C2H3NaO2.3(H2O)
Appearance: white to colourless crystals or powder
Chinese Factory Supply Top Purity Sodium acetate trihydrate 6131-90-4 Fast Delivery
- Molecular Formula:C2H3NaO2.3(H2O)
- Molecular Weight:136.08
- Appearance/Colour:white to colourless crystals or powder
- Melting Point:58 °C
- Boiling Point:117.1 °C at 760 mmHg
- PKA:4.76 (acetic acid)(at 25℃)
- Flash Point:40 °C
- PSA:67.82000
- Density:1,45 g/cm3
- LogP:-1.43670
Sodium acetate trihydrate(Cas 6131-90-4) Usage
|
Chemical Properties |
Sodium acetate trihydrate is the trihydrate sodium salt oaf acetic acid. It is a colorless, odorless crystals that can be weathered in the air. Soluble in water and ether, pH of 0.1M aqueous solution is 8.9. moderately soluble in ethanol, 5.3 g/100mL. Anhydrous Sodium acetate occurs as colorless, transparent crystals or a granular crystalline powder with a slight acetic acid odor. |
|
Preparation |
Sodium acetate is prepared by reacting sodium hydroxide or sodium carbonate with acetic acid in aqueous solution. The solution is evaporated to obtain hydrated crystals of sodium acetate. NaOH + CH3COOH → CH3COONa + H2O Na2CO3 + CH3COOH → 2CH3COONa + CO2 + H2O |
|
General Description |
Sodium acetate trihydrate is a crystalline compound obtained through the crystallization of sodium acetate in water. |
| Uses |
Sodium acetate trihydrate plays a versatile role in buffering systems, nucleic acid purification, protein crystallization, and various industrial applications, showcasing its significance in chemistry, pharmaceuticals, and food preservation. sed as a buffer in the pH range of 4.0 to 6.0. |
|
Biochem/physiol Actions |
Sodium acetate trihydrate (SAT) is a phase change material (PCM) that can be easily combined with the preparation of domestic hot water, space heating, solar heating, and radiant floor heating systems. The hydrated salt of SAT possesses high latent heat density and thus can be used for low-temperature heat storage. |
|
Safety |
Sodium acetate is widely used in cosmetics, foods, and pharmaceutical formulations, and is generally regarded as a nontoxic and nonirritant material. A short-term feeding study in chickens with a diet supplemented with 5.44% sodium acetate showed reduced growth rates that were attributed to the sodium content.(9) Sodium acetate is poisonous if injected intravenously, is moderately toxic by ingestion, and is an irritant to the skin and eyes. LD50 (rat, oral): 3.53 g/kg LD50 (mouse, IV): 0.38 g/kg LD50 (mouse, SC): 8.0 g/kg |
|
storage |
Sodium acetate should be stored in airtight containers. |
|
Incompatibilities |
Sodium acetate reacts with acidic and basic components. It will react violently with fluorine, potassium nitrate, and diketene. |
|
Regulatory Status |
GRAS listed. Accepted as a food additive in Europe. Included in the FDA Inactive Ingredients Database (injections, nasal, otic, ophthalmic, and oral preparations). |
InChI:InChI=1/C2H4O2.Na.3H2O/c1-2(3)4;;;;/h1H3,(H,3,4);;3*1H2/q;+1;;;/p-1
6131-90-4 Process route
-
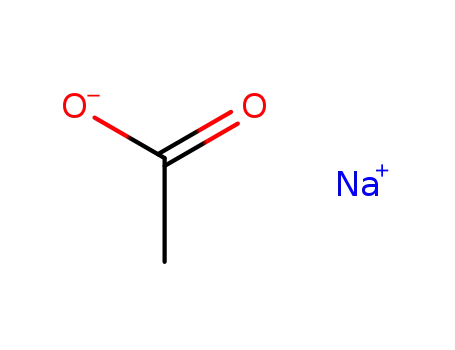
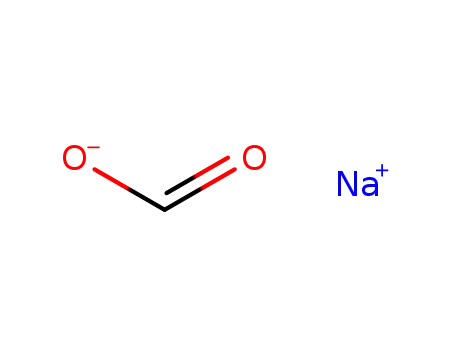
- 127-09-3
sodium acetate

-
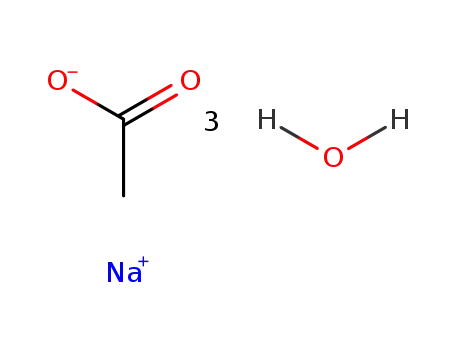
- 6131-90-4
sodium acetate trihydrate
| Conditions | Yield |
|---|---|
|
With water; In neat (no solvent); formation of supersaturated soln. via H2O uptake, solidification after contact with solid materials and slow formation of NaCH3CO2*3H2O;;
|
|
|
With water; in moist air at ambient temp.;;
|
|
|
With H2O; in moist air at ambient temp.;;
|
|
|
With H2O; In neat (no solvent); formation of supersaturated soln. via H2O uptake, solidification after contact with solid materials and slow formation of NaCH3CO2*3H2O;;
|
-
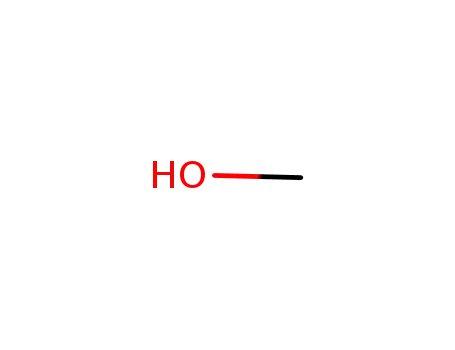
- 67-56-1
methanol

-
- 141-53-7
sodium formate

-

- 6131-90-4
sodium acetate trihydrate
| Conditions | Yield |
|---|---|
|
With water; In neat (no solvent); heating NaHCO2 in autoclave to 200 to 300°C unnder pressure in presence of little H2O while treating with CH3OH vapor;;
|
|
|
With H2O; In neat (no solvent); heating NaHCO2 in autoclave to 200 to 300°C unnder pressure in presence of little H2O while treating with CH3OH vapor;;
|
|
|
With water; In neat (no solvent); heating NaHCO2 in autoclave to 200 to 300°C unnder pressure in presence of little H2O while treating with CH3OH vapor;;
|
6131-90-4 Upstream products
-
64-19-7
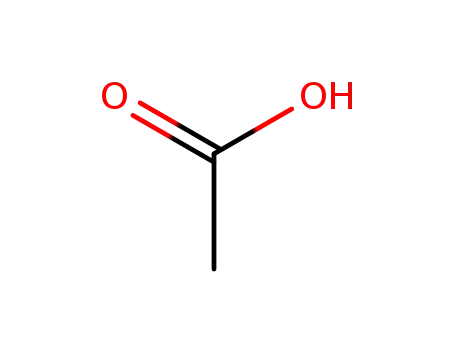
acetic acid
-
127-09-3

sodium acetate
-
7732-18-5
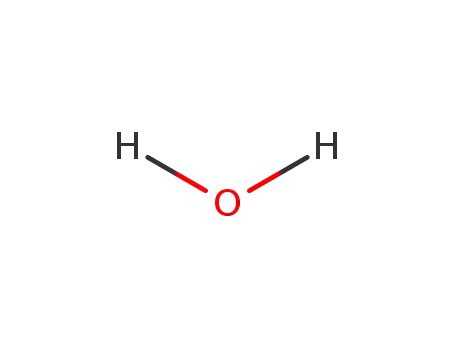
water
-
7647-14-5

sodium chloride
6131-90-4 Downstream products
-
74-84-0
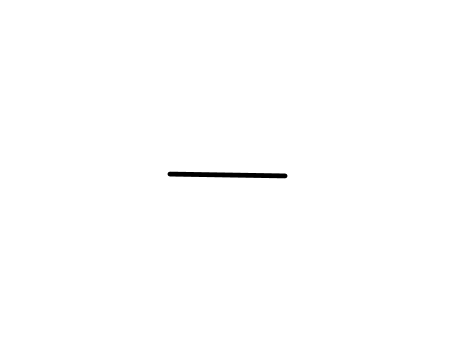
ethane
-
74-85-1
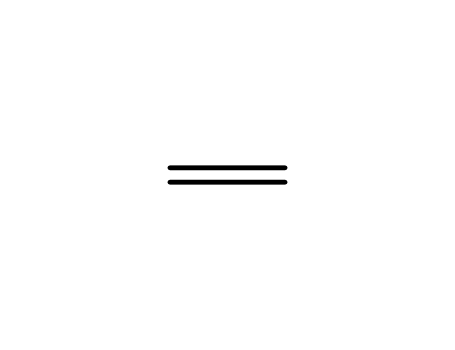
ethene
-
108-24-7
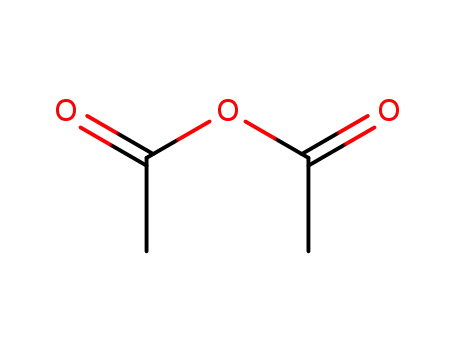
acetic anhydride
-
138555-65-4
[(2,6-(dichloro)-4-(methylsulfonyl)-phenyl)hydrazono]propanedinitrile
Relevant Products
-
Sodium carboxymethyl cellulose
CAS:9004-32-4
-
Sodium tetraborate
CAS:1330-43-4
-
IGF-1 LR3
CAS:946870-92-4