151533-22-1
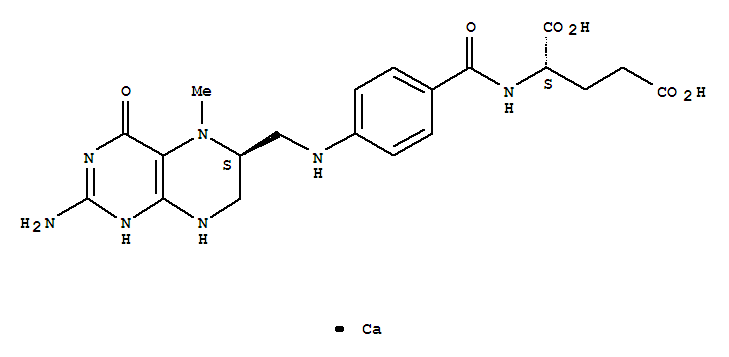
- Product Name:Levomefolate calcium
- Molecular Formula:C20H23CaN7O6
- Purity:99%
- Molecular Weight:497.52
Product Details;
CasNo: 151533-22-1
Molecular Formula: C20H23CaN7O6
Appearance: Off-white to pale yellow solid
Buy Quality Top Purity Levomefolate calcium 151533-22-1 Safe Shipping
- Molecular Formula:C20H23CaN7O6
- Molecular Weight:497.52
- Appearance/Colour:Off-white to pale yellow solid
- Melting Point:>300 °C
- PSA:208.43000
- LogP:-1.67900
Levomefolate calcium(Cas 151533-22-1) Usage
|
Description |
Levomefolate calcium is a synthetic form of folate, an essential B-vitamin required for various physiological processes in the body. It is synthesized as a stable crystalline form of the calcium salt, making it suitable for use as a supplement. |
|
Uses |
Treatment of Birth Defects: Levomefolate calcium is essential for preventing birth defects, particularly neural tube defects like spina bifida, by providing the necessary folate supplementation during pregnancy. Megaloblastic Anemia: Levomefolate calcium is effective in treating megaloblastic anemia, a condition characterized by abnormally large red blood cells. This type of anemia often results from folate deficiency. Depression: Levomefolate calcium has been utilized as an antidepressant. Folate plays a role in neurotransmitter synthesis and regulation, and supplementation may help alleviate depressive symptoms, particularly in individuals with folate deficiency. |
InChI:InChI=1/C20H25N7O6.Ca/c1-27-12(9-23-16-15(27)18(31)26-20(21)25-16)8-22-11-4-2-10(3-5-11)17(30)24-13(19(32)33)6-7-14(28)29;/h2-5,12-13,22H,6-9H2,1H3,(H,24,30)(H,28,29)(H,32,33)(H4,21,23,25,26,31);/q;+2/p-2/t12-,13-;/m0./s1
151533-22-1 Relevant articles
The combined oral contraceptive pill containing drospirenone and ethinyl estradiol plus levomefolate calcium
Rachel B Rapkin , MD &Mitchell D Creinin , MD
Expert Opinion on Pharmacotherapy, Volume 12, 2011 - Issue 15
A new oral contraceptive containing drospirenone and ethinyl estradiol plus levomefolate calcium was formulated to decrease the risk of neural tube defects in pregnancies conceived while taking or shortly after discontinuing this pill.
Bioequivalence study of an oral contraceptive containing ethinylestradiol/drospirenone/levomefolate calcium relative to ethinylestradiol/drospirenone and to levomefolate calcium alone
Hartmut Blode a, Christine Klipping b, Frank Richard c, Dietmar Trummer a, Beate Rohde a, Konstanze Diefenbach a
, Contraception Volume 85, Issue 2, February 2012, Pages 177-184
Forty-four subjects received in an intraindividual crossover design single doses of the new tablet formulation or the established ethinylestradiol/drospirenone tablet or the levomefolate calcium tablet.
Relevant Products
-
Monosodium glutamate
CAS:32221-81-1
-
Epitalon
CAS:307297-39-8
-
Sodium carbonate
CAS:497-19-8